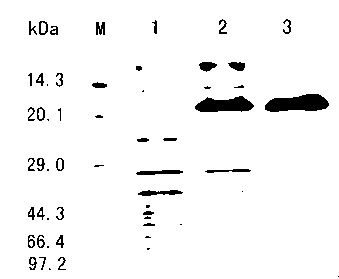Recombinant ribose-5-phosphate isomerase and application thereof
A phosphate isomerase and ribose technology, applied in the field of bioengineering, can solve the problems of excessive generation of by-products, non-reusable substrates, complex purification steps, etc., and achieves the effect of good heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Construction of recombinant expression vector pET22-tlRpib and acquisition of genetic engineering strain BL21(DE3) / pET22-tlRpib
[0036] Synthetic with NdeI / XhoI restriction siteThermotoga lettingae The ribose-5-phosphate isomerase gene 435bp was ligated into pET-22b (+) to obtain the pET22-tlRpib plasmid.
[0037] Add 2 μL pET22-tlRpib plasmid to 100 μL BL21 competent cell suspension, and mix gently.
[0038] Let it stand in an ice bath for 30 minutes, evenly spread the LB plate containing 100 μg / mL ampicillin, and culture it upside down at 37°C for 12-15 hours to obtain a positive clone, named recombinant Escherichia coli BL21(DE3) / pET22-tlRpib.
[0039] Sequencing of the recombinant plasmid pET22-tlRpib showed that the inserted fragment was a 435bp DNA fragment encoding a protein consisting of 145 amino acids.
Embodiment 2
[0040] The acquisition of embodiment 2 recombinant tl-Rpib enzyme
[0041] The single clone of the genetically engineered strain BL21(DE3) / pET22-tlRpib was picked to the LB seed medium containing 50 μg / mL of ampicillin, and cultured with shaking at 200 rpm at 37°C for 12 hours.
[0042] The seed culture solution was added to the fermentation LB medium at a ratio of 1:50 and cultured at 37°C until OD600=0.8-2.0, and IPTG was added to a final concentration of 0.1mM.
[0043] Incubate at 100 rpm at 28°C for 6 hours, and collect the bacterial liquid. The cells were collected by centrifugation at 10,000 rpm at 4°C for 10 min, the supernatant was discarded, and washed 3 times with normal saline.
[0044] According to the ratio of 1g bacteria: 20mL equilibration buffer, add buffer to sonicate cells, supernatant is centrifuged and passed through Chelating Sepharose Fast Flow affinity medium chromatography column, low imidazole elution buffer elutes impurity protein, high imidazole el...
Embodiment 3
[0050] Example 3 The influence of pH on the enzyme activity of recombinant tl-Rpib
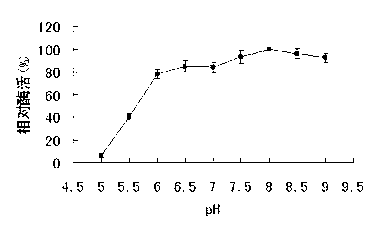
[0051] The substrate D-psicose concentration was 50mM, the reaction temperature was 65°C, and the reaction time was 15min. Three kinds of buffer systems, citrate buffer (50mM, pH 5-5.5), phosphate buffer (50 mM, pH 6.0 -7.5) and Tris hydrochloric acid buffer (50 mM, pH 8.0-9.0) were reacted. The optimal pH value of the recombinant tl-Rpib enzyme is 8, and 80% of the maximum enzyme activity can be retained between pH 6-9, see image 3 .
[0052] Dissolve the recombinant tl-Rpib enzyme powder in phosphate buffer solution with pH values of 6, 7, and 8 respectively, and place it at room temperature for a certain period of time, adjust the pH of the enzyme solution to 8, and adjust the volume to detect its enzyme activity. Figure 4 The results showed that the recombinant tl-Rpib enzyme had good stability at pH 8, and kept 60% of the enzyme activity after 24 hours at room temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com