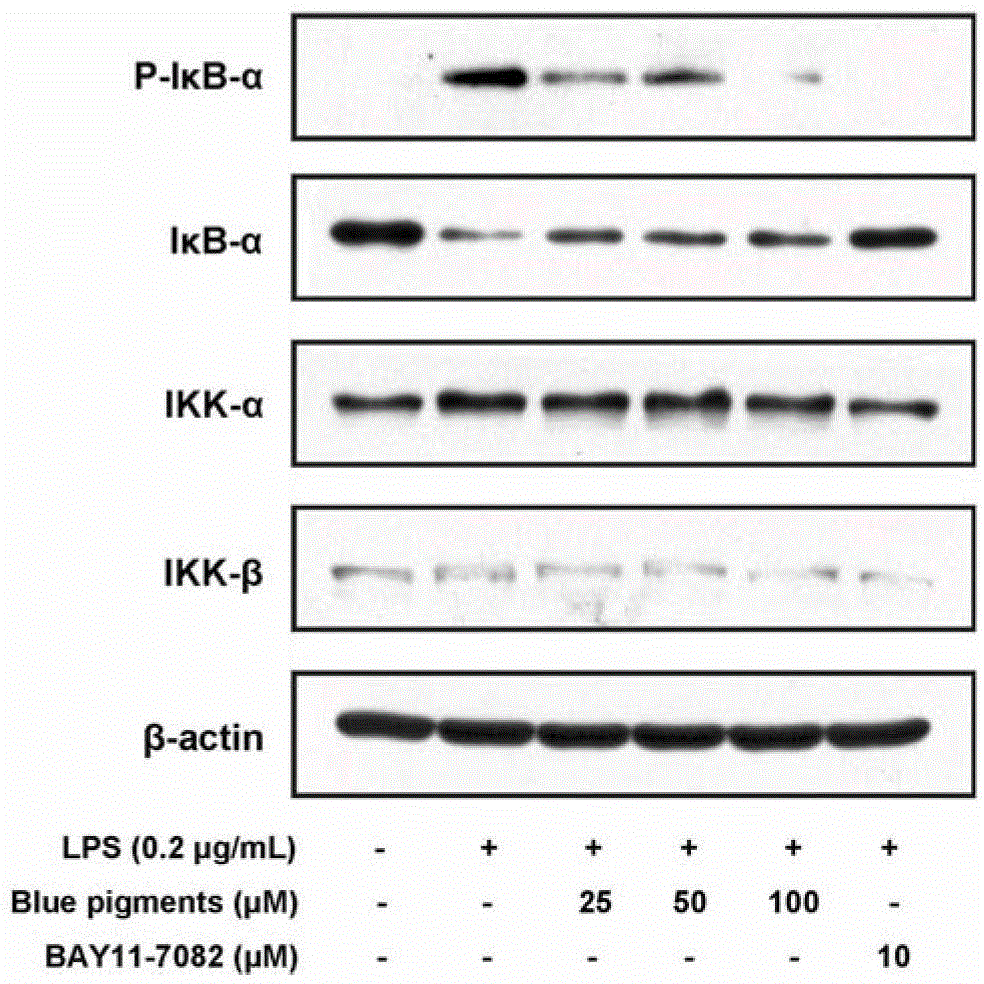
Application of genipin amino acid derivative as NF-kappa B inhibitor
An amino acid and derivative technology, which is applied in the application field of genipin amino acid derivatives as NF-κB inhibitors, and achieves the effects of good medicinal prospects, easy promotion and remarkable curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0259] Example 1 Preparation of Genipin Amino Acid Derivatives
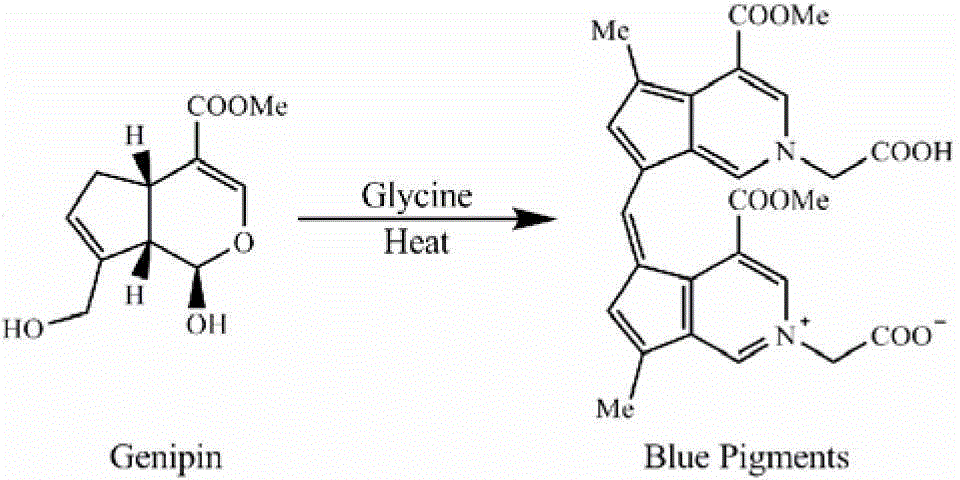
[0260] Genipin amino acid derivatives were prepared according to the method of Fujikama et al. (Fujikama S, Fukui Y, Koga K, Kumada J. (1987) Brilliant skyblue pigment formation from Gardenia fruits. Journal of Fermentation Technology 65:419-424).
[0261] Add 8.8mmol of genipin and 8.8mmol of amino acid to 400mL of PBS (100mmol, pH7.0) solution successively, stir at 80°C for 4h, then pass the obtained genipin amino acid derivative through HP-20 macroporous resin column , separate, and collect the eluate with an absorbance value of 590nm, and dry the collected eluate with a freeze dryer to obtain the finished product of genipin amino acid derivative. The obtained genipin amino acid derivatives are sealed and kept in cold storage at 2-8°C until use.
[0262] Genipin can react with amino acids containing primary amino groups to generate genipin amino acid derivatives of the present invention, such as alanine, vali...
Embodiment 2
[0263] Example 2 Preparation of Genipin Glycine Derivatives
[0264] Add 8.8mmol of genipin and 8.8mmol of glycine to 400mL of PBS (100mmol, pH7.0) solution successively, stir at 80°C for 4h, then pass the obtained genipin glycine derivative through HP-20 macroporous resin column , using a mixed solution of ethyl acetate, methanol, and water as the eluent for elution and separation, wherein the volume ratio of ethyl acetate-methanol-water in the eluent is: 8:3:1, and the eluate is collected. And carry out freeze dryer drying to obtain the finished product of genipin glycine derivative. The obtained genipin glycine derivatives are stored in a refrigerator and sealed at 2-8°C until use.
Embodiment 3
[0265] Example 3 Preparation of Genipin Tyrosine Derivatives
[0266] Add 8.8mmol of genipin and 8.8mmol of tyrosine to 400mL of PBS (100mmol, pH7.0) solution successively, stir at 80°C for 4h, then pass the obtained genipin tyrosine derivative through HP-20 Macroporous resin column, using a mixed solution of ethyl acetate, methanol, and water as the eluent for elution and separation, wherein the volume ratio of ethyl acetate-methanol-water in the eluent is: 8:3:1, collected The eluate is dried in a freeze dryer to obtain the finished product of genipin tyrosine derivative. The obtained genipin tyrosine derivatives are stored in a refrigerator and sealed at 2-8°C until use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com