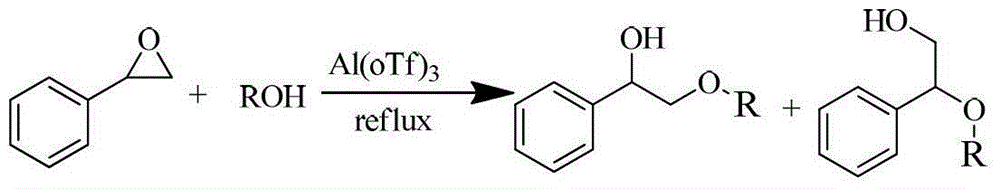
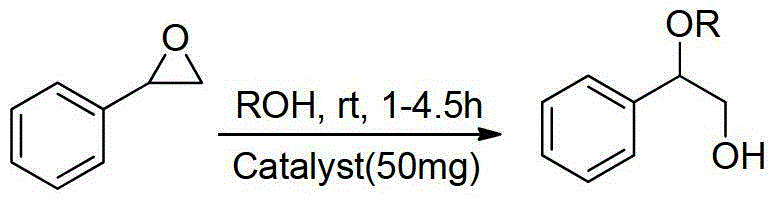
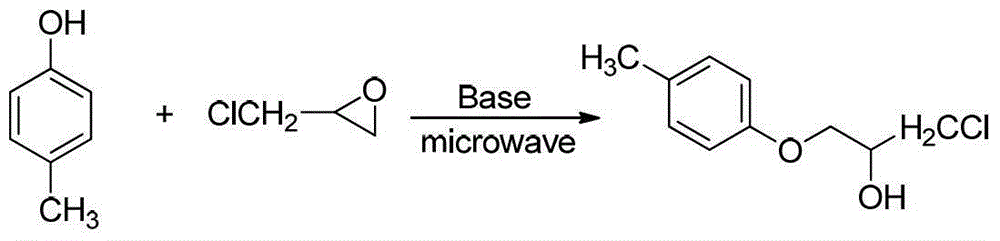
Beta-alkoxy alcohol compound synthesis method
The technology of an alkoxy alcohol and a synthesis method is applied in the field of organic synthesis of organic intermediates, can solve the problems of high high temperature and high pressure risk factor, high yield of target compounds and high production cost, and achieves short reaction time, low production cost and high yield. rate increase effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the synthesis of 1-phenoxy-2-propanol (1), the synthetic route is:
[0032]
[0033] Add propylene oxide (0.012mol, 0.696g, analytically pure, Shanghai Jingchun Chemical Reagent Co., Ltd.), phenol (0.01mol, 0.94g, analytically pure, Shanghai Jingchun Chemical Reagent Co., Ltd.) and pre-prepared (C 4 h 12 N 2 ) 2 [BiCl 6 ]Cl H 2 O (0.0001mol, 0.05g), stirred and reacted at room temperature for 30min, then filtered, washed and dried to recover the catalyst, and the filtrate was added with 20mlH 2 Diluted with O, then extracted 3 times with 60ml ethyl acetate (analytical grade, Sinopharm Reagent), and the organic phase was sequentially washed with 20ml saturated NaHCO 3 solution, 20mlH 2 O, 20ml saturated NaCl solution washing, anhydrous NaCl 2 SO 4 Dry, remove ethyl acetate, and then separate by column chromatography (EA:PE=1:9 / 1:8 / 1:7 / 1:6) to obtain 1.40 g of the main product A.
[0034] 1-phenoxy-2-propanol: yield 92.1%, 1 H NMR(CDCl3)δ7.22-7...
Embodiment 2
[0035] Embodiment 2: Synthesis of 1-chloro-3-(4-methylphenoxy)-2-propanol (2), the synthetic route is:
[0036]
[0037] Add epichlorohydrin (0.012mol, 1.11g, analytically pure, Shanghai Jingchun Chemical Reagent Co., Ltd.), 4-methylphenol (0.01mol, 1.08g, analytically pure, Shanghai Jingchun Chemical Co., Ltd.) in a 25ml single-necked flask successively. Reagent Co., Ltd.) and pre-prepared (C 4 h12 N 2 ) 2 [BiCl 6 ]Cl H 2 O (0.0001mol, 0.05g), stirred and reacted at room temperature for 40min, then filtered, washed and dried to recover the catalyst, and the filtrate was added with 20mlH 2 Diluted with O, then extracted 3 times with 60ml ethyl acetate (analytical grade, Sinopharm Reagent), and the organic phase was sequentially washed with 20ml saturated NaHCO 3 solution, 20mlH 2 O, 20ml saturated NaCl solution washing, anhydrous NaCl 2 SO 4 Drying, removal of ethyl acetate, followed by column chromatography (EA:PE=1:9 / 1:8 / 1:7 / 1:6) afforded 1.80 g of a single produc...
Embodiment 3
[0039] Embodiment 3: Synthesis of 1-chloro-3-(1-naphthyloxy)-2-propanol (3), the synthetic route is:
[0040]
[0041] In a 25ml single-necked flask, add epichlorohydrin (0.012mol, 1.11g, analytically pure, Shanghai Jingchun Chemical Reagent Co., Ltd.), naphthol (0.01mol, 1.44g, analytically pure, Shanghai Jingchun Chemical Reagent Co., Ltd.) ) and pre-prepared (C 4 h 12 N 2 ) 2 [BiCl 6 ]Cl H 2 O (0.0001mol, 0.05g), stirred and reacted at room temperature for 50min, then filtered, washed and dried to recover the catalyst, and the filtrate was added with 20mlH 2 Diluted with O, then extracted 3 times with 60ml ethyl acetate (analytical grade, Sinopharm Reagent), and the organic phase was sequentially washed with 20ml saturated NaHCO 3 solution, 20mlH 2 O, 20ml saturated NaCl solution washing, anhydrous NaCl 2 SO 4 Drying, removal of ethyl acetate, followed by column chromatography (EA:PE=1:9 / 1:8 / 1:7 / 1:6) yielded 2.10 g of a single product C.
[0042] 1-chloro-3-(1-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com