Technological method for preparing boric acid, calcium carbonate and sodium nitrate by decomposing ulexite mine with nitric acid
A technology of sodium borate stone and process method, which is applied in the field of chemical engineering disciplines, can solve problems such as environmental pollution and waste of resources, and achieve the effects of low temperature, low corrosion, and easy reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
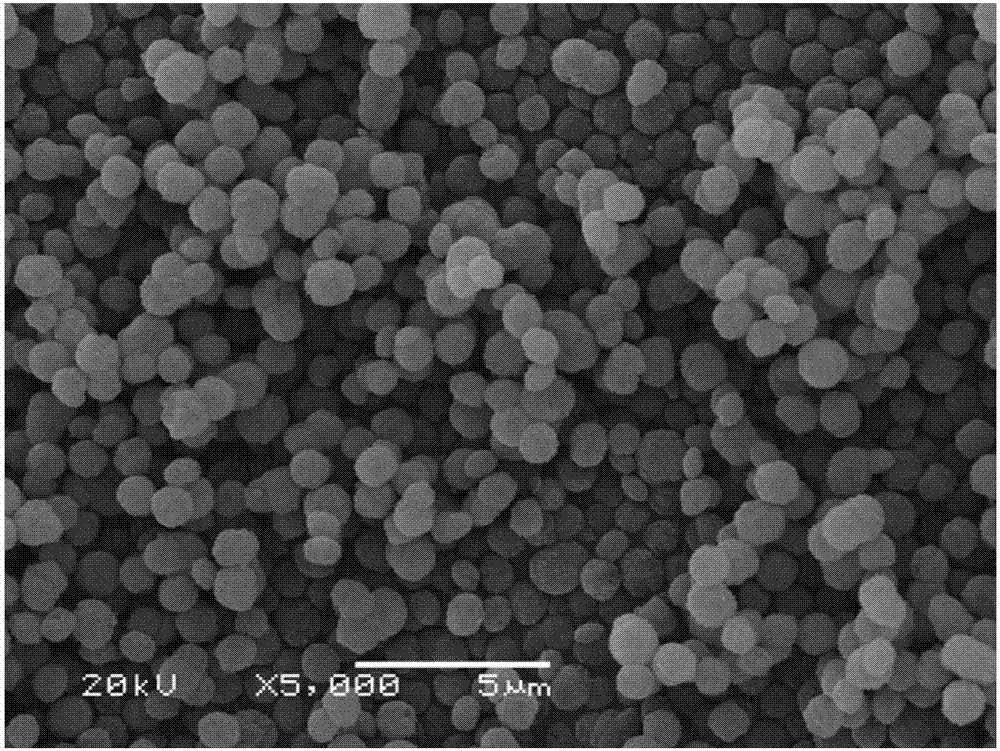
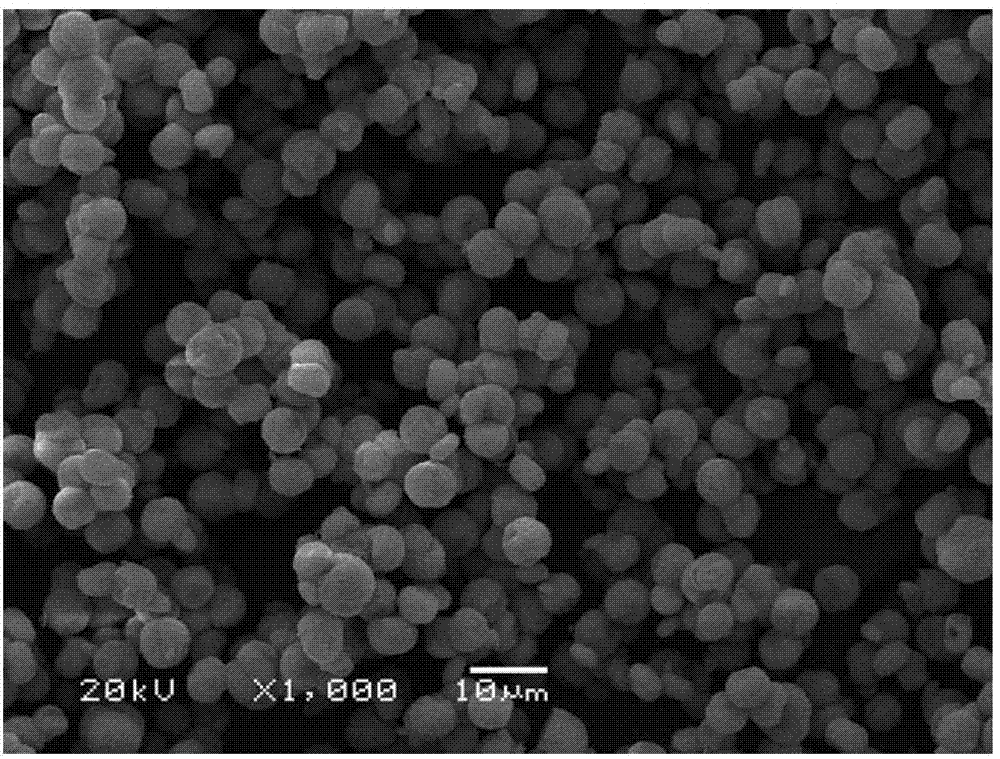
[0022] Composition 250kg into B 2 o 3 31%, CaO11.81%, Na 2 O6.90% and Cl4.6% sodium borate ore is ground on a ball mill for 0.5h, and the ore powder screened by a 100-mesh standard sieve is placed in a 2m 3 In the acid hydrolysis reaction tank, add 0.77m 3 Boric acid washing water, 0.40m 3 Sodium nitrate mother liquor and 0.080m 3 68% concentrated nitric acid, acid hydrolysis reaction at liquid-solid ratio 5:1 and 70°C for 40 minutes, cooled to 30°C to crystallize, and separated to obtain 82.6kg of boric acid and 1.1m of boric acid mother liquor 3 ;At 30°C, add 56kg of sodium carbonate to the boric acid mother liquor, precipitate for 30 minutes, and obtain 51.1kg of spherical calcium carbonate; evaporate the mother liquor of separated calcium carbonate to 40 Baume degrees, cool down to 40°C for crystallization, and separate 90kg of nitric acid by flotation Sodium product, 13.7kg crude boric acid and 0.40m 3 Mother liquor, crude boric acid and mother liquor return to acid...
Embodiment 2
[0024] Composition 240kg into B 2 o 3 33%, CaO12.01%, Na 2 O7.50% and Cl5.60% sodium borate ore was ground on a ball mill for 1.5 hours, and the ore powder sieved by a 200-mesh standard sieve was placed in a 2m 3 In the acid hydrolysis reaction tank, add 0.87m 3 Boric acid washing water, 0.50m 3 Sodium nitrate mother liquor and 0.072m 3 68% concentrated nitric acid, after acid hydrolysis reaction at liquid-solid ratio 6:1 and 50°C for 50 minutes, cool down to 30°C to crystallize and separate boric acid 87.6kg and boric acid mother liquor 1.25m 3 ;At 50°C, add 60kg of sodium carbonate to the boric acid mother liquor, precipitate for 50 minutes, and obtain 49.7kg of spherical calcium carbonate; evaporate the mother liquor of separated calcium carbonate to 45 degrees Baume, cool down to 35°C to crystallize, and flotation to separate 87.5kg Sodium nitrate product, 14.2kg crude boric acid and 0.50m 3 Mother liquor, crude boric acid and mother liquor return to acid hydrolysis ...
Embodiment 3
[0026] Composition 200kg into B 2 o 3 39.00%, CaO13.27%, Na 2 O5.61% and Cl0.60% 120-mesh sodium borate concentrate is placed in 2m 3 In the acid hydrolysis reaction tank, add 0.36m 3 Boric acid washing water, 0.35m 3 Sodium nitrate mother liquor and 0.089m 3 68% concentrated nitric acid, after acid hydrolysis at 4:1 liquid-solid ratio and 60°C for 80 minutes, cool down to 25°C to crystallize and separate boric acid 98.6kg and boric acid mother liquor 0.69m 3 ;At 40°C, add 55kg of sodium carbonate to the boric acid mother liquor, precipitate for 40 minutes, and obtain 47.1kg of spherical calcium carbonate; evaporate the mother liquor of separated calcium carbonate to 37 Baume degrees, cool down to 30°C for crystallization, and separate 102kg of nitric acid by flotation Sodium product, 12.5kg crude boric acid and 0.35m 3 Mother liquor, crude boric acid and mother liquor return to acid hydrolysis batching.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com