Method for synthesizing 1,2-orthodiol through immobilized type heteropolyacid phase-transfer catalytic oxidation
A technology of phase-transfer catalysis and phase-transfer catalyst, applied in chemical instruments and methods, preparation of organic compounds, preparation of oxidation reaction, etc., can solve the problems of serious environmental pollution, inability to use industrial production, low yield, etc., and achieve reaction operation Simple, mild reaction conditions, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
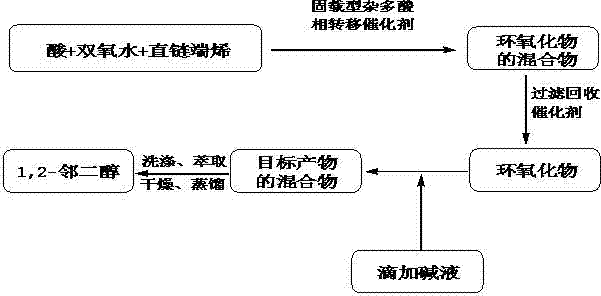
[0020] The first step: add formic acid 46g, hydrogen peroxide 3.4g, polystyrene resin catalyst [π-C 5 h 5 N(CH 2 ) 15 CH 3 ] 3 [PMoW 3 o 24 ] 0.07g, 7g of 1-hexene, control the reaction temperature at 50°C, keep the reaction mixture solution for 4h, filter and recover the catalyst after the reaction, and obtain the epoxide mixture;
[0021] Step 2: Add alkali solution dropwise to the epoxide mixture so that the pH of the mixture is 10, and react at 40°C for 2 hours to obtain the target product mixture;
[0022] The third step: after the reaction, the target product mixture is extracted with ethyl acetate, the extract is washed twice with saturated brine, and the washed extract is washed with anhydrous Na 2 SO 4 Drying and dehydration, vacuum filtration and distillation under reduced pressure, 9.2 g of the product 1,2-hexanediol was distilled out, with a yield of 94%. The purity of 1,2-hexanediol was 98.2% as detected by gas chromatography.
Embodiment 2
[0024] The first step: add formic acid 46g in four-necked flask, hydrogen peroxide 34g, polystyrene resin catalyst [(C 4 h 9 ) 4 N] 3 PMo 12 o 40 4.9g, 98g of 1-heptene, control the reaction temperature at 80°C, keep the reaction mixture solution for 3h, filter and recover the catalyst after the reaction, and obtain the epoxide mixture;
[0025] Step 2: Add alkali solution dropwise to the epoxide mixture so that the pH of the mixture is 12, and react at 70°C for 2 hours to obtain the target product mixture;
[0026] The third step: after the reaction, the target product mixture is extracted with ethyl acetate, the extract is washed twice with saturated brine, and the washed extract is washed with anhydrous Na 2 SO 4 Drying and dehydration, vacuum filtration and distillation under reduced pressure, the product 1,2-heptanediol was evaporated to 115g, with a yield of 87.1%; the purity of 1,2-heptanediol was 98.7% as detected by gas chromatography.
Embodiment 3
[0028] The first step: add formic acid 46g in four-necked flask, hydrogen peroxide 2g, polystyrene resin catalyst [(C 4 h 9 ) 4 N] 3 PW 12 o 40 0.5g, 6.6g of 1-octene, control the reaction temperature at 40°C, keep the reaction mixture solution for 6h, filter and recover the catalyst after the reaction, and obtain the epoxide mixture;
[0029] Step 2: Add an alkali solution dropwise to the epoxide mixture to make the pH of the mixture 11, and react at 60°C for 4 hours to obtain the target product mixture;
[0030] The third step: after the reaction, the target product mixture is extracted with ethyl acetate, the extract is washed twice with saturated brine, and the washed extract is washed with anhydrous Na 2 SO 4 Drying and dehydration, vacuum filtration and distillation under reduced pressure, 7.3g of the product 1,2-octanediol was distilled out, with a yield of 84.9%; the purity of 1,2-octanediol was 99.0% as detected by gas chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com