Preparation method of 9,10-di(4-hydroxy alkoxy alcohol-phenyl)-phenanthrene aromatic diol
A technology for hydroxyalkoxy alcohol and diol, which is applied in the synthesis field of aromatic diol, can solve the problems of unpredictable overall performance of materials, increased glass transition temperature, low melt strength, etc., and achieves inhibition of oxidation The effect of generating carbonyl defects and increasing the glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
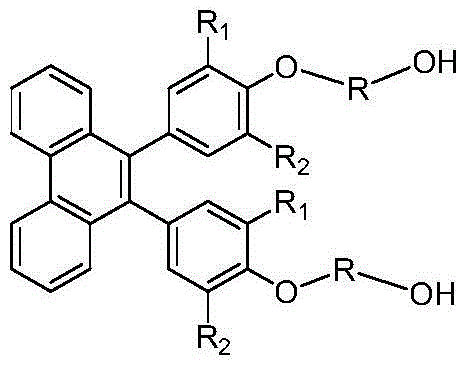
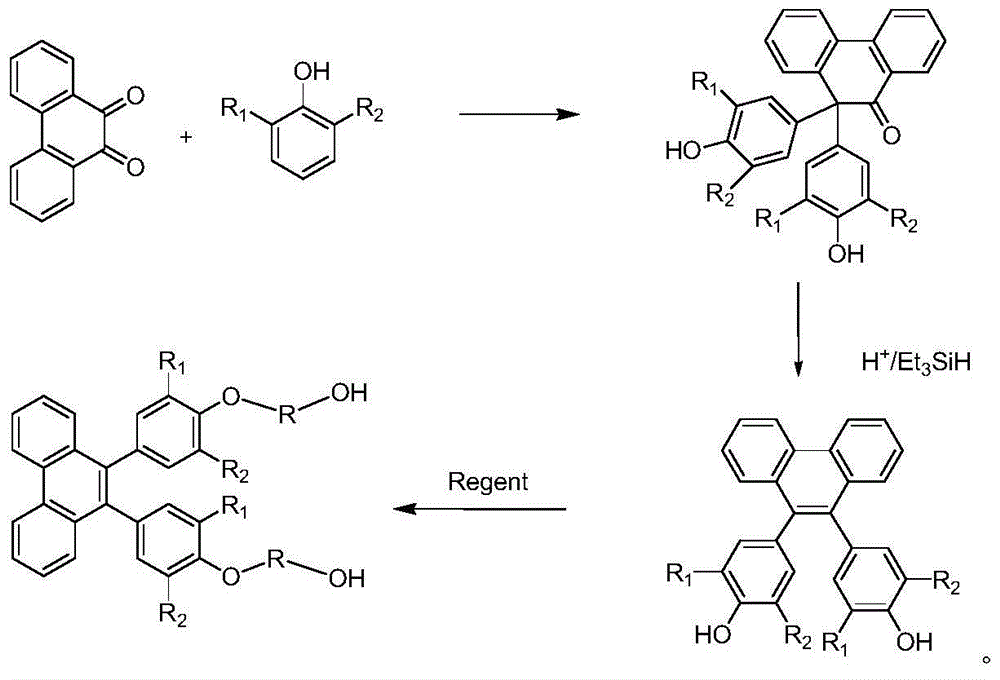
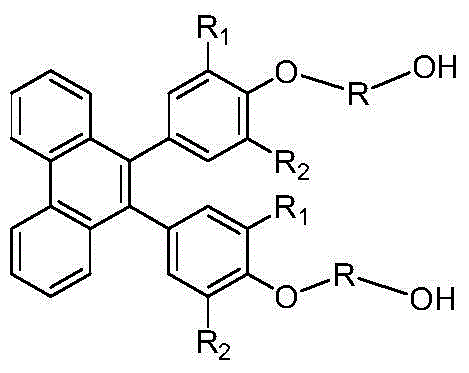
[0024] The invention provides a preparation method of 9,10-bis(4-hydroxyalkoxy alcohol-phenyl)-phenanthrene aromatic dihydric alcohol and its derivatives, using phenanthrenequinone and phenol as raw materials, dehydrating under acid catalysis , to obtain the structure of 10,10'-bis(4-hydroxy-phenyl)-phenanthrene pinaconone; on the action of reducing agent and dehydrating agent, 9,10-di (4-Hydroxy-phenyl)-phenanthrene pinacol structure; finally, reacting this structure with halohydrin or alkylene oxide derivatives or enol or aliphatic dihydric alcohols and their derivatives to prepare 9, 10-bis(4-hydroxyalkoxy alcohol-phenyl)-phenanthrene aromatic diol and its derivatives, the specific structure is as follows:
[0025]
[0026] Among them, the substituent R 1 ~R 2 Respectively selected from hydrogen, alkyl, alkoxy and aromatic groups and corresponding derivative structures, straight-chain or branched alkyl or alkoxy groups with n=1-12 in R or fatty acids containing ether b...
Embodiment 1
[0048]
[0049] Under the condition of nitrogen protection, add reaction raw materials phenanthrenequinone (0.1mol) and phenol (or 0.6mol), catalyst methanesulfonic acid (0.6mol) and solvent CCl 4 (2L). Stirring was started and the temperature continued to rise to 80°C. After reacting for 3 hours, cool to room temperature and filter. The obtained filter cake was washed with detergent to remove most impurities and color, and recrystallized with ethanol to obtain colorless transparent crystals. 1 H NMR (500MHz, CDCl 3 ): δ=8.02(d,1H), 7.80(d,1H), 7.70(d,1H), 7.65(t,1H), 7.55-7.08(m,10H), 6.7(t,1H), 6.0( d,1H,), 5.5(m,2H).
Embodiment 2
[0051]
[0052] Under the condition of nitrogen protection, add the product of Reaction Example 1 (0.1 mol), reducing and dehydrating agent triethyl silicon (0.12 mol), catalyst dehydration and solvent 500ml of trifluoroacetic acid into the reactor. Start stirring and continue to heat up to reflux for 12 hours. After cooling to room temperature, pour into ice water and stir until completely melted. The filter cake obtained by filtration is washed with water, and colorless transparent crystals are obtained by recrystallization, column chromatography or sublimation methods. The yield is about 70%. 1 H NMR (500MHz, CDCl 3 ): δ=7.80(d,2H), 7.70(d,2H), 7.62(t,2H), 7.55-7.08(m,10H), 5.3(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com