Micronized tinidazole powder injection
A technology of tinidazole powder and tinidazole, which is applied in the field of medicine, can solve the problems of uneven freeze-dried powder, poor clarity, and low product purity, and achieve improved bioavailability, water solubility, and clinical efficacy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
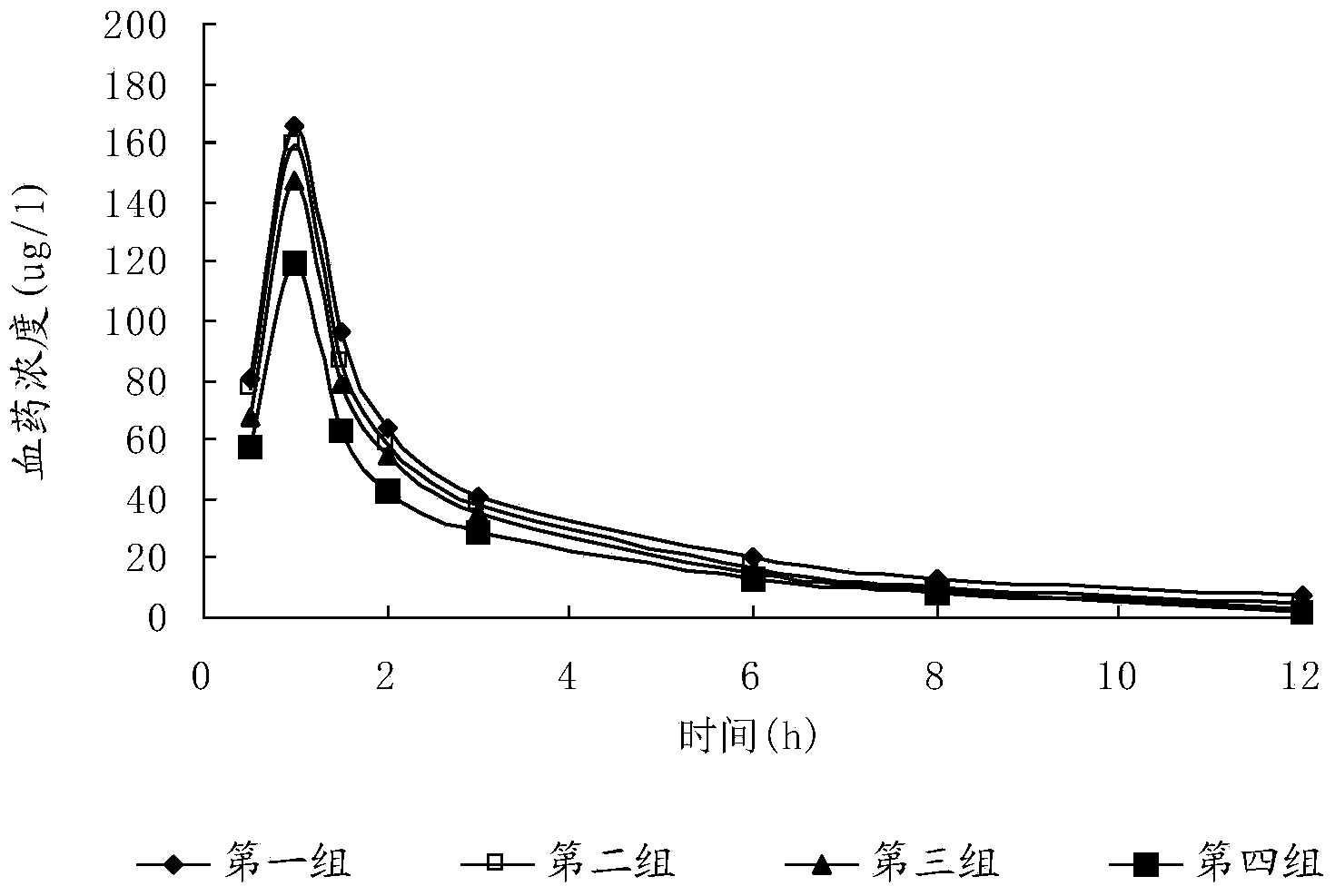
Image
Examples
Embodiment 2
[0037] The preparation of the micronized injection tinidazole powder preparation of embodiment 2
[0038] (1) 100g of tinidazole, 300g of polyethylene glycol 400, 600g of mannitol, and 20g of citric acid were mixed and dissolved, and freeze-dried in a large plate to obtain sterile powder of tinidazole for injection;
[0039] (2) Pre-crushing the sterile powder of tinidazole for injection with a fluid energy mill using collision technology to make coarse particles with a particle size of 70 μm;
[0040] (3) Use the CWJ-30 ultrafine pulverizer to ultrafinely pulverize the above coarse particles and pulverize them into 2-10μm fine powder;
[0041]Grinding conditions: the air temperature after freeze-drying is 5°C, the water content is 0.5%, the pressure when injected into the ultrafine pulverizer is 1.2MPa, the working pressure of the ultrafine pulverizer is 1.0MPa, the internal working temperature is 3°C, and the pulverization time is 120min.
[0042] (4) Subpackaging under as...
Embodiment 3
[0043] The preparation of the micronized injection tinidazole powder preparation of embodiment 3
[0044] (1) 100g of tinidazole, 400g of propylene glycol, 700g of mannitol, and 20g of citric acid were mixed and dissolved, and freeze-dried in a large plate to obtain sterile powder of tinidazole for injection;
[0045] (2) The fluid energy mill using collision technology pre-crushed the sterile powder of tinidazole for injection to make coarse particles with a particle size of 100 μm;
[0046] (3) Use the CWJ-30 ultrafine pulverizer to ultrafinely pulverize the above coarse particles and pulverize them into 2-10μm fine powder;
[0047] Grinding conditions: the air temperature after freeze-drying is 8°C, the water content is 0.4%, the pressure when injected into the ultrafine pulverizer is 1.0MPa, the working pressure of the ultrafine pulverizer is 1.2MPa, the internal working temperature is 6°C, and the pulverization time is 150min.
[0048] (4) Subpackaging under aseptic con...
Embodiment 4
[0049] The preparation of the micronized injection tinidazole powder preparation of embodiment 4
[0050] (1) 100 g of tinidazole was pre-crushed aseptic tinidazole powder for injection using a fluid energy mill with collision technology to make coarse particles with a particle size of 120 μm;
[0051] (2) Use the CWJ-30 ultrafine pulverizer to ultrafinely pulverize the above coarse particles and pulverize them into 2-10μm fine powder;
[0052] Grinding conditions: the air temperature after freeze-drying is 7°C, the water content is 0.7%, the pressure when injected into the ultrafine pulverizer is 0.9MPa, the working pressure of the ultrafine pulverizer is 1.3MPa, the internal working temperature is 4°C, and the pulverization time is 70min.
[0053] (3) Mix and dissolve micronized tinidazole, 200g polyethylene glycol 400, 300g propylene glycol, 800g mannitol, and 20g citric acid, freeze-dry in a large plate, and obtain tinidazole sterile powder for injection;
[0054] (4) Su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com