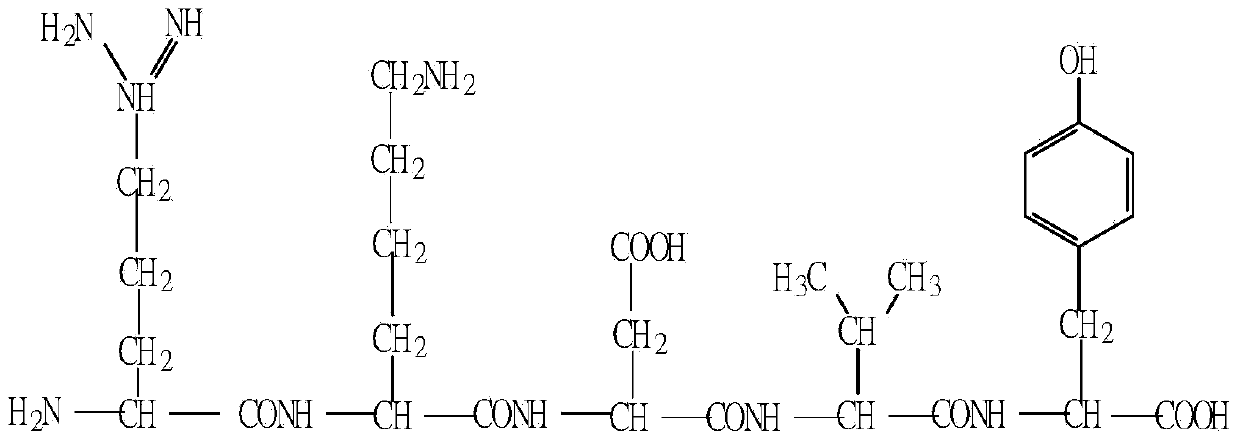
Application of thymopentin in preparation of drug for treating patient with severe hepatitis b
A technology for thymus and severe disease, applied in the field of medicine, can solve the problems of lack of 50mg product specification, restricted application, etc., to achieve the effect of facilitating administration, improving compliance, and meeting special needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of Thymopentin (50 mg) for large dose injection:
[0044] 1), solution preparation:
[0045] In a class 10,000 clean room, take the prescribed amount of thymopentin 50,000mg and mannitol 50,000mg, add it to about 900ml of water for injection, shake well, and dilute to 1,000ml to get ready.
[0046] 2), endotoxin monitoring
[0047] Samples were taken for limulus test, and the endotoxin content per milliliter was less than 2.5 EU.
[0048] 3), content monitoring
[0049]Using high performance liquid chromatography (Chinese Pharmacopoeia 2010 Edition, Appendix ⅤD), samples were taken to detect the content of thymopentin, and the results showed that the content of thymopentin in the sample was 90% to 110% of the labeled amount. , indicating that the quality standard is met.
[0050] 4), filter sterilization
[0051] Under Class 100 clean conditions, use a 0.2 μM filter membrane to filter and sterilize.
[0052] 5), filling
[0053] Under the ...
Embodiment 2
[0059] Example 2, detection of the effect of Thymopentin (50 mg) for large dose injection:
[0060] 1. Check
[0061] 1. pH
[0062] Test method: pH value determination method (Chinese Pharmacopoeia 2010 edition two appendix VI H)
[0063] Measuring process: 3 batches of 50 mg thymopentin prepared in Example 1 were randomly taken, and the batch numbers were named 20090102, 20090103 and 20090104, each was dissolved with 5 ml of water, and the pH value of the solution was measured according to the law.
[0064] Results: The pH values of the three batches of test samples were all between 6.0 and 8.0. The measurement data are shown in Table 1.
[0065] Table 1 Thymopentin Alkalinity Determination Results for Injection
[0066]
[0067] 2. Solution clarity and color
[0068] Test method: Take 5 tubes of this product, add 1ml of water to dissolve, the solution should be colorless and clear; if it is cloudy, compare it with the No. 1 turbidity standard solution (Appendix ...
Embodiment 3
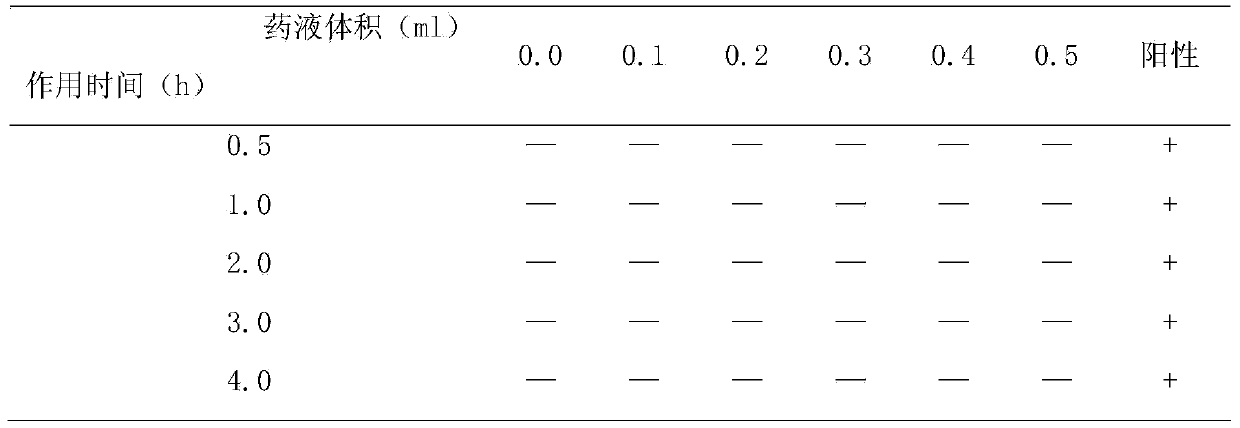
[0120] Embodiment 3, the hemolysis test of Thymopentin for injection:
[0121] 1. Experimental materials
[0122] 1.1. Test product
[0123] Name: Thymopentin for injection prepared in Example 1 of the present invention (Thymopentin for Iniection). Batch number: 20090102. Specifications: 50mg / bottle. Physical and chemical properties: white powder. Stability and storage: airtight, store in a cool and dark place.
[0124] 1.2. Experimental animals
[0125] Species: Rabbit. Department: Big-eared White Rabbit. Gender and number: female, 1 only. Animal age: 16 weeks. Animal weight: 2.5 kg. Animal source: Beijing Keyu Animal Breeding Center. Animal certificate number: SCXK (Beijing) 20070003.
[0126] 2. Experimental methods and index testing requirements
[0127] 2.1. Experimental method
[0128] Thymopentin for injection: During the test, 0.9% sodium chloride injection was prepared into 3 concentrations of 0.1mg / ml, 1.0mg / ml, and 3.3mg / ml.
[0129] Preparation of bl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com