Method for detecting phadodendrol in cosmetics
A technique for rhododendrol and cosmetics, applied in the field of cosmetic analysis, can solve the problem of rhododendrol detection being in a blank state and the like, and achieve the effects of simple and effective sample pretreatment, avoiding peak broadening and accurate detection results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
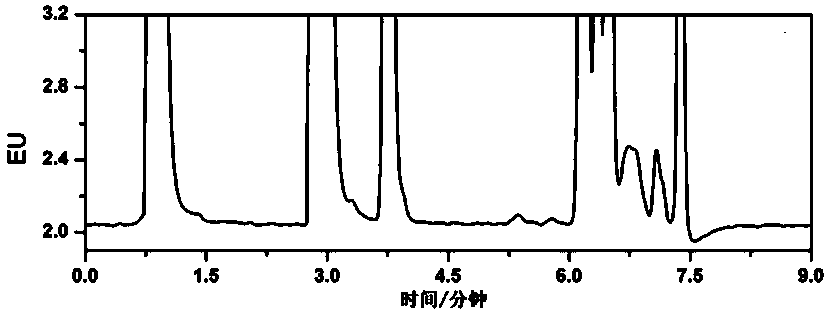
Image
Examples
Embodiment 1
[0040] Embodiment 1 establishes rhododendron alcohol standard curve
[0041] Dissolve 50 mg rhododendron alcohol (purity ≥ 85%) in acetonitrile to prepare a standard stock solution with a concentration of 1000 μg / mL. Pipette the standard stock solution and prepare standard working solutions with concentrations of 15, 50, 100, 250, 500, and 1000 mg / L with formic acid-acetonitrile containing 0.1% by volume respectively, and take the standard working solutions of each concentration, After filtering through a 0.22 μm filter membrane, take 10 μL of injection samples for determination according to liquid chromatography conditions. Each series of samples is injected 3 times, and the average peak area value is taken.
[0042] Liquid chromatography conditions:
[0043] High-performance liquid chromatography equipped with a fluorescence detector (Agilent 1260, Agilent, USA);
[0044] Chromatographic column: Phenomenex Kinetex C18, 100 × 4.6mm (inner diameter), 2.6 μm (particle size); ...
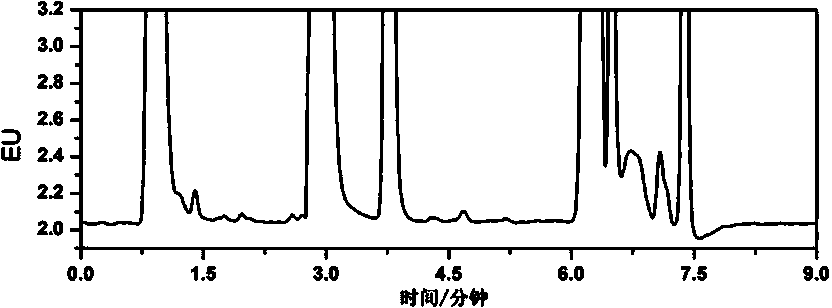
Embodiment 2
[0052] Example 2 Detection of rhododendron in cosmetics
[0053] (1) Sample handling
[0054] Weigh 0.20 g of the sample to be tested (accurate to 0.001 g), put it in a 10 mL plastic centrifuge tube, add 0.15 mL of 0.02 mol / L ammonium acetate aqueous solution, vortex for 30 s, and add 5% trichloroacetic acid-acetonitrile to mix Extraction (70:30, V / V) 1.85 mL, vortexed for 60 s, ultrasonically extracted for 15 min, then centrifuged at 10,000 rpm for 10 min, the supernatant was passed through a 0.22 μm microporous membrane to obtain the extract solution, Standby; the above-mentioned samples to be tested are respectively cream, lotion, water and facial mask.
[0055] (2) Liquid chromatography conditions
[0056] High-performance liquid chromatography equipped with a fluorescence detector (Agilent 1260, Agilent, USA);
[0057] Chromatographic column: Phenomenex Kinetex C18, 100 × 4.6mm (inner diameter), 2.6 μm (particle size);
[0058] Mobile phase: A: formic acid aqueous sol...
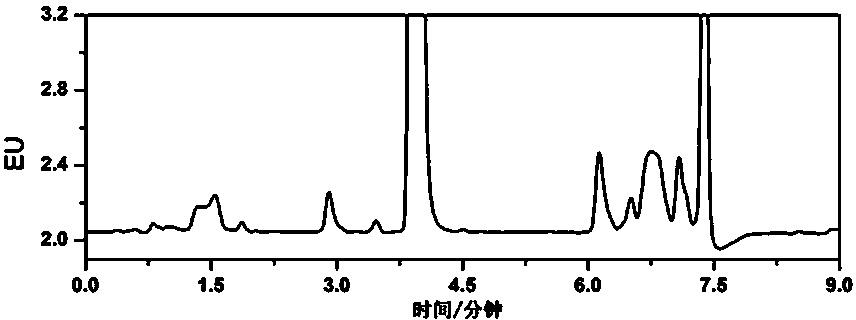
Embodiment 3
[0067] Example 3 Detection of rhododendol in cosmetics with a concentration of 1 mg / kg rhododendron
[0068] (1) Sample handling
[0069] Using the same cream, lotion, lotion and facial mask as the base in Example 2, prepare a test sample of cream, lotion, lotion and facial mask containing rhododendol at a concentration of 1 mg / kg.
[0070] Weigh 0.20 g of the sample to be tested (accurate to 0.001 g), put it in a 10 mL plastic centrifuge tube, add 0.15 mL of 0.02 mol / L ammonium acetate aqueous solution, vortex for 30 s, and add 5% trichloroacetic acid-acetonitrile to mix Extraction (70:30, V / V) 1.85 mL, vortexed for 60 s, ultrasonically extracted for 15 min, then centrifuged at 10,000 rpm for 10 min, the supernatant was passed through a 0.22 μm microporous membrane to obtain the extract solution, spare.
[0071] (2) Liquid chromatography conditions
[0072] High-performance liquid chromatography equipped with a fluorescence detector (Agilent 1260, Agilent, USA);
[0073] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com