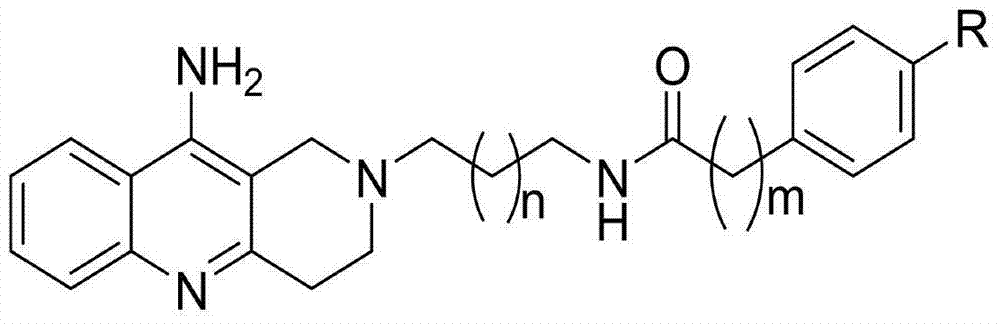
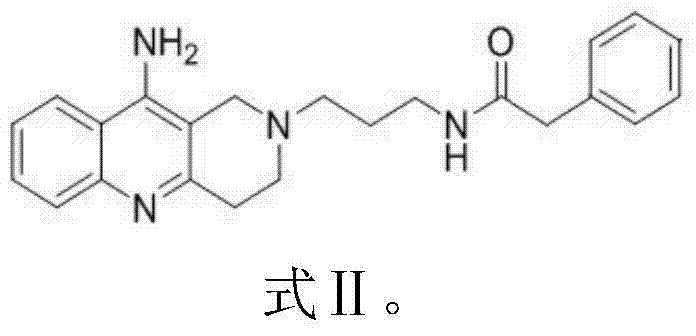
Aza-acridine aromatic ring derivative as well as preparation method and application thereof
A technology of azaacridine and aromatic ring, which is applied in the field of azaacridine aromatic ring derivatives and its preparation, can solve the problems of toxic side effects and high treatment cost, and achieve low cytotoxicity, low cytotoxicity and important economic value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of Azaacridine Aromatic Ring Derivative T1
[0037] 1) Synthesis of intermediate compound 1:
[0038] 3.5 mL of 4-benzylpiperidone and 20 mL of phosphorus oxychloride (POCl 3 ) was added to a 100mL round-bottomed flask, and after stirring for 10min, the anthranilic Add 3 g to the above reaction solution, stir and reflux for 2 hours; stop heating, cool to room temperature, slowly pour the reaction solution into a beaker filled with crushed ice, let stand for 3 to 5 minutes, stir, and slowly drop into the beaker Add ammonia water, adjust the pH to 8-9, a large amount of yellow precipitates are formed, stop dripping ammonia water, filter with suction, and obtain yellow precipitates; use petroleum ether: acetone = 12:1 system to separate through the column, and use acetone to recrystallize to obtain light Yellow crystal intermediate compound 1;
[0039] Compound 1, 1H NMR (400MHz, CDCl 3 )δ8.11(d, J=8.4Hz, 1H), 7.98(d, J=8.4Hz, 1H), 7.65(t, J=7.7Hz, ...
Embodiment 2
[0063] Example 2 Synthesis of Azaacridine Aromatic Ring Derivative T3
[0064] 1) Synthesis of intermediate compound 1:
[0065] 3.5 mL of 4-benzylpiperidone and 20 mL of phosphorus oxychloride (POCl 3 ) into a 100mL round bottom flask, stirred for 10min, then added 3g of anthranilic acid into the above reaction solution, stirred and refluxed for 2h; stopped heating, cooled to room temperature, slowly poured the reaction solution into a container filled with crushed ice In the beaker, let it stand for 3-5 minutes and then stir, slowly add ammonia water to the beaker, adjust the pH to 8-9, a large amount of yellow precipitate is formed, stop dripping ammonia water, filter with suction, and get a yellow precipitate; use petroleum ether : The system of acetone=12:1 is separated by column, and recrystallized with acetone to obtain light yellow crystal intermediate compound 1;
[0066] Compound 1, 1H NMR (400MHz, CDCl 3 )δ8.11(d, J=8.4Hz, 1H), 7.98(d, J=8.4Hz, 1H), 7.65(t, J=7.7...
Embodiment 3
[0090] Example 3 Synthesis of Azaacridine Aromatic Ring Derivative T11
[0091] 1) Synthesis of intermediate compound 1:
[0092] 3.5 mL of 4-benzylpiperidone and 20 mL of phosphorus oxychloride (POCl 3 ) into a 100mL round bottom flask, stirred for 10min, then added 3g of anthranilic acid into the above reaction solution, stirred and refluxed for 2h; stopped heating, cooled to room temperature, slowly poured the reaction solution into a container filled with crushed ice In the beaker, let it stand for 3-5 minutes and then stir, slowly add ammonia water to the beaker, adjust the pH to 8-9, a large amount of yellow precipitate is formed, stop dripping ammonia water, filter with suction, and get a yellow precipitate; use petroleum ether : The system of acetone=12:1 is separated by column, and recrystallized with acetone to obtain light yellow crystal intermediate compound 1;
[0093] Compound 1, 1H NMR (400MHz, CDCl 3 )δ8.11(d, J=8.4Hz, 1H), 7.98(d, J=8.4Hz, 1H), 7.65(t, J=7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com