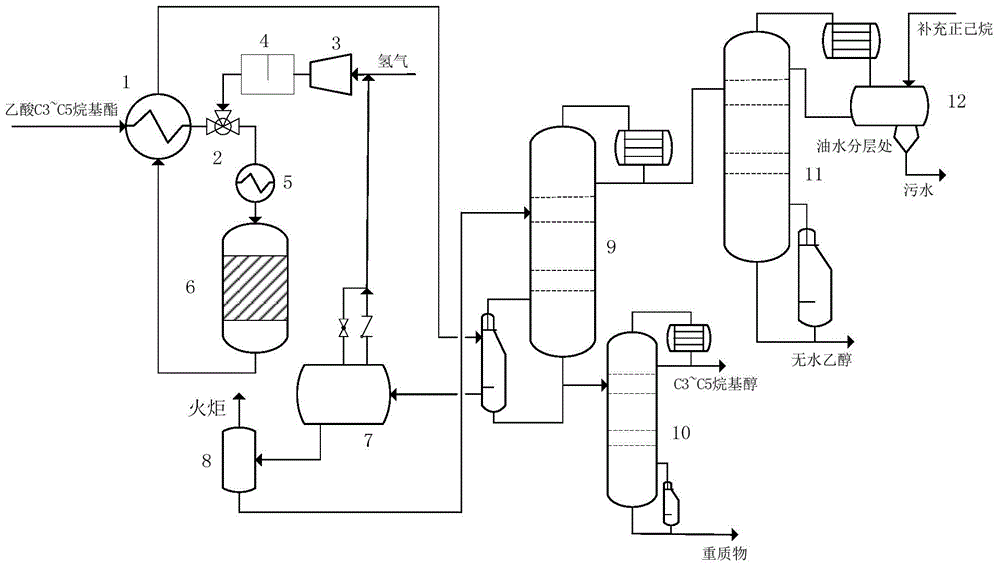
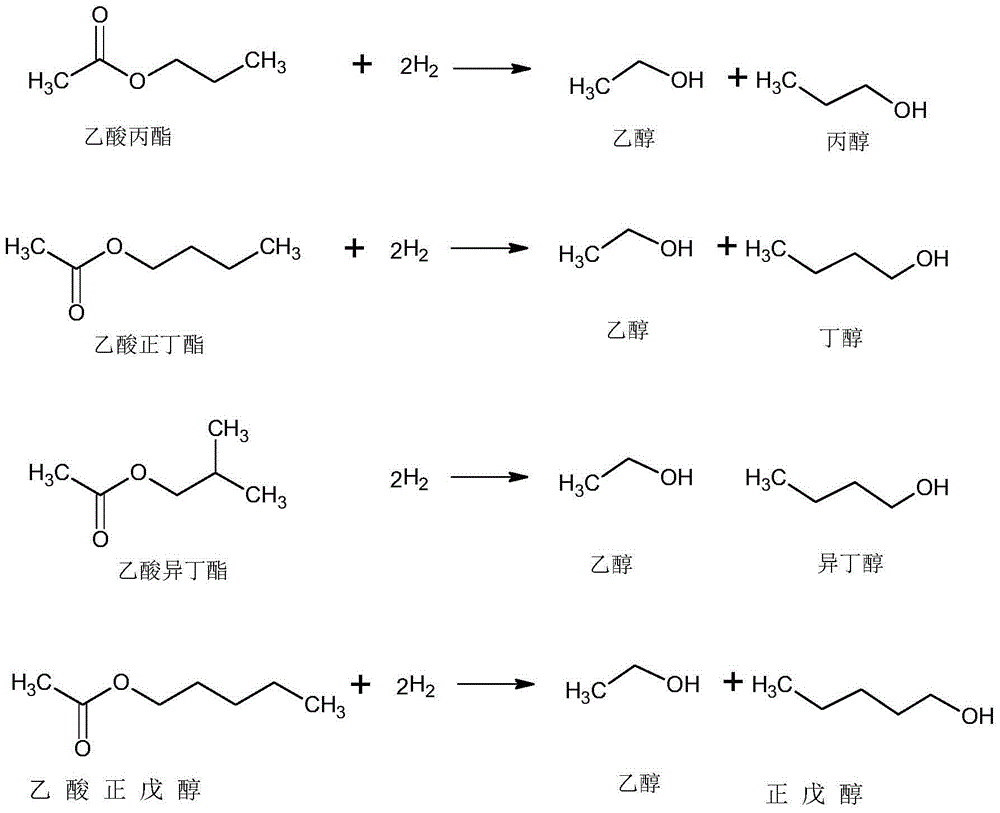
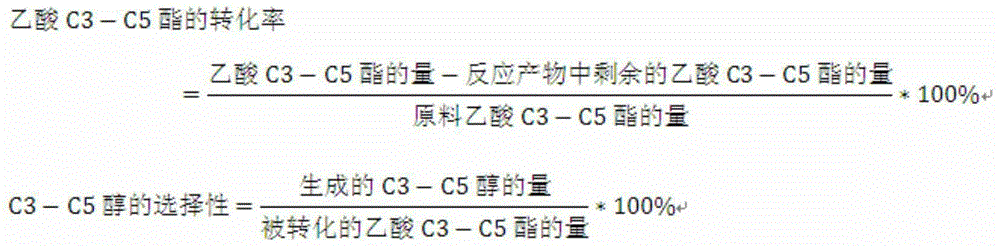
Technology producing C3-C5 alkyl alcohol and co-producing alcohol by adding hydrogen in acetic acid C3-C5 alkyl ester
A C3-C5, alkyl ester technology, applied in the field of hydrogenation of C3-C5 alkyl acetate to produce C3-C5 alkyl alcohol and ethanol production, can solve the problem of high hydrogenation reaction pressure, harsh equipment requirements, sec-butyl problems such as low alcohol selectivity, to achieve the effect of high heat utilization rate, simple and practical process, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Preparation of hydrogenation catalyst: prepared according to copper-chromium (Cu-Cr) atomic ratio of 2.35. Take copper nitrate (Cu(NO 3 ) 2 ·3H 2 O) 70g and chromium nitrate (Cr(NO 3 ) 3 9H 2 O) 50g were dissolved in water respectively at 50°C to form a mixed aqueous solution. Weigh 12g carrier γ-Al 2 o 3 Add it to the solution, and maintain it in an incubator at 25°C for 20h. Ordinary filtration was used to remove excess solution, and the filtrate was dried in an oven at 120°C for 12 hours, and then calcined in a muffle furnace for 5 hours at 500°C to obtain an alumina-supported copper-chromium catalyst.
[0050] (2) Hydrogenation catalyst loading and reduction: 5 parts by weight of the copper-chromium catalyst and 1 part by weight of alumina particles were mixed uniformly and loaded into the reactor. The hydrogenation reactor is a small stainless steel fixed bed reactor. The inner diameter of the reaction chamber is 14mm, filled with 30g of the catalyst ...
Embodiment 2
[0053] The catalyst preparation steps are the same as in Example 1, except that the active component copper-chromium (Cu-Cr) atomic ratio is 10. Under optimized process conditions, the conversion rate of sec-butyl acetate is 97.62%, and the selectivity of sec-butanol is 98.33%.
Embodiment 3
[0055] The catalyst preparation steps are the same as in Example 1, except that the active component copper-silver (Cu-Ag) atomic ratio is 18, and the carrier is mesoporous molecular sieve. Under optimized process conditions, the conversion rate of sec-butyl acetate is 93.61%, and the selectivity of sec-butanol is 97.21%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com