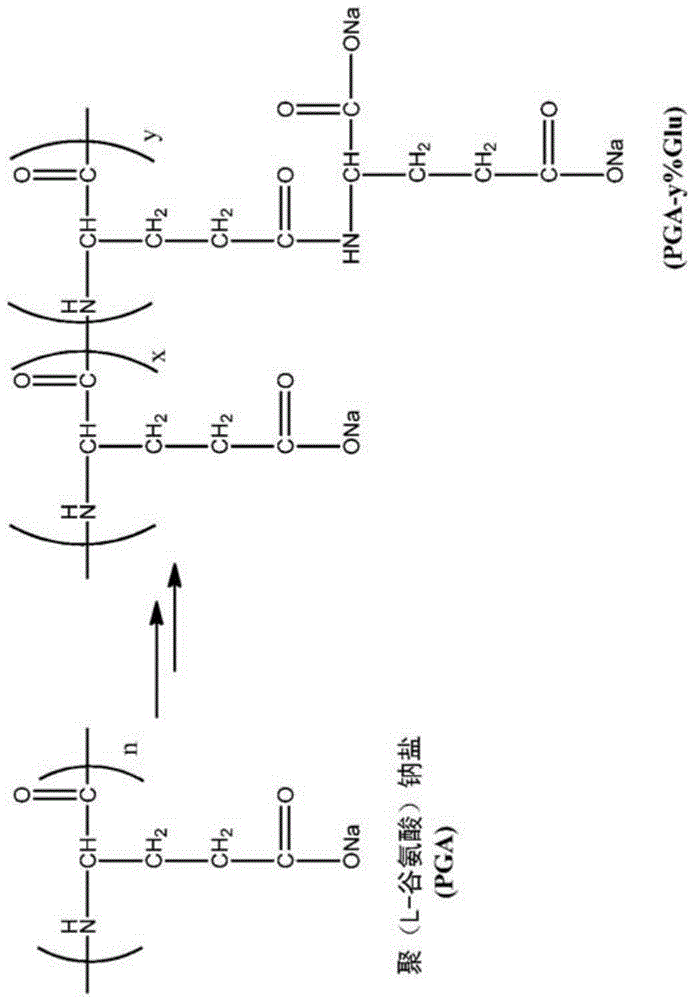
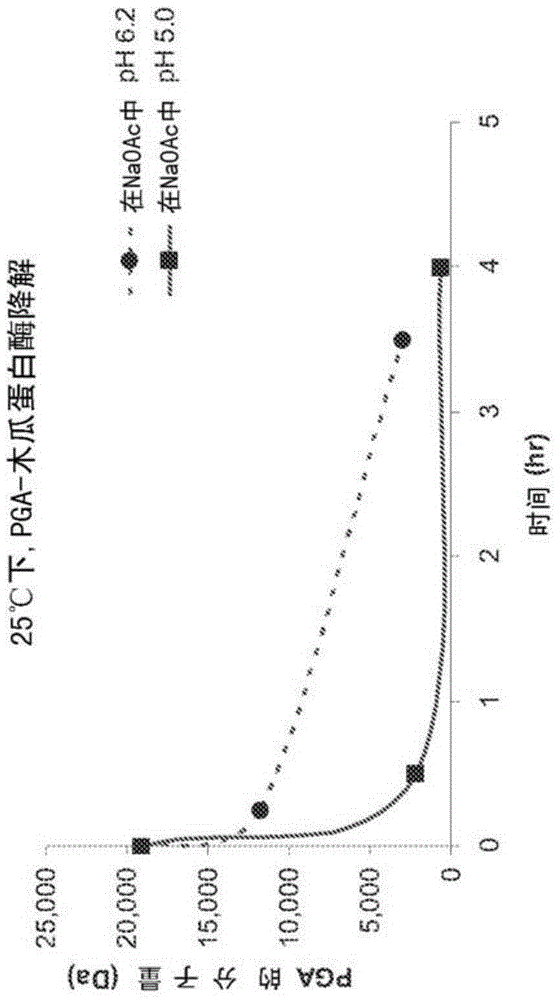
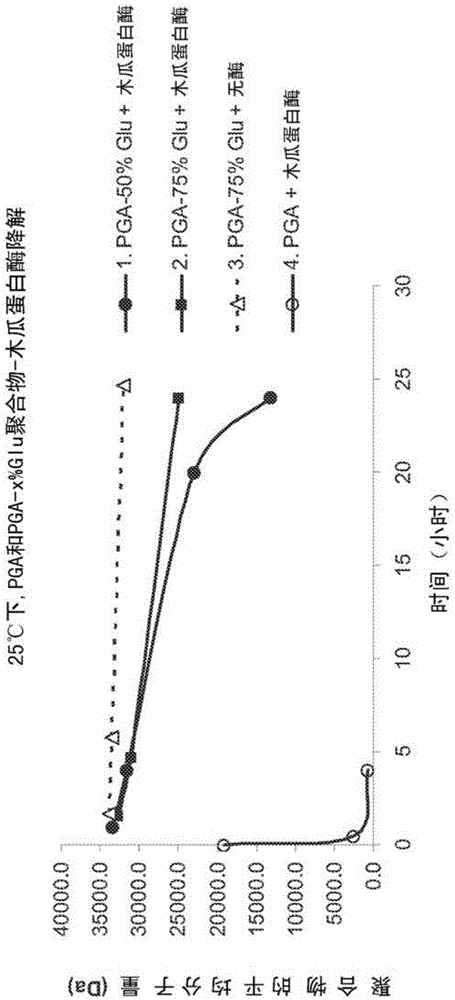
Co-polymer conjugates
A technology of polymers and conjugates, which can be used in drug combinations, medical preparations of inactive ingredients, digestive systems, etc., and can solve problems such as poor bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] If the preparation of the pharmaceutical formulation involves intimate mixing of a pharmaceutical excipient with the active ingredient in salt form, it may be desirable to use non-basic pharmaceutical excipients, ie acidic or neutral excipients.
[0079] In some embodiments, the pharmaceutical composition may comprise one or more physiologically acceptable surface active substances, carriers, diluents, excipients, lubricants, suspending agents, film-forming substances and coatings. Clothing adjuvants, or combinations of these substances; and compounds disclosed herein (eg, polymer conjugates described herein). Carriers or diluents acceptable for therapeutic use are well known in the pharmaceutical art and are described, for example, in Remington's Pharmaceutical Sciences, 18th Ed., Mack Publishing Co., Easton, PA (1990), which is incorporated herein by reference in its entirety. Preservatives, stabilizers, dyes, sweeteners, fragrances, flavoring agents, etc. may be prov...
Embodiment 1
[0122]
[0123] Can be prepared according to the general route shown in Example 1 containing the structure (Q) Polymer conjugates of repeating units of formula (III).
Embodiment 2
[0125] Partial PGGA Esters - 10% Q
[0126] To PGA-OH (200 mg), EDC (446 mg, 2.33 mmol) and HOAt (317 mg, 2.33 mmol) weighed into an oven-dried vial (40 mL) with a magnetic bar was added 15 mL of anhydrous DMSO. The reaction mixture was stirred at ambient temperature for 2 hours. Glu-diester.HCl (413 mg, 1.40 mmol) and DIEA (243 μL, 1.40 mmol) were added. The mixture was stirred at ambient temperature for 2 h, NH 3 / dioxane (6.2 mL, 0.5M), and the reaction mixture was stirred overnight (16 hours) at ambient temperature. The mixture was slowly poured into 0.2N aqueous HCl to precipitate the polymer. The precipitate was washed with water (2x) and the polymer was isolated by centrifugation. The resulting polymer was frozen and lyophilized to constant weight. Yield 83.3% (446.8mg), GPC (MW: 69.55kDa).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com