A kind of method for preparing sodium chromate by acid-dissolved oxidation of chromite
A technology of iron ore acid and sodium chromate, applied in the direction of chromate/dichromate, etc., can solve the problems of environmental secondary pollution, general separation effect, separation of trivalent chromium, etc., and achieve improved recycling and process flow Short, the effect of improving the dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
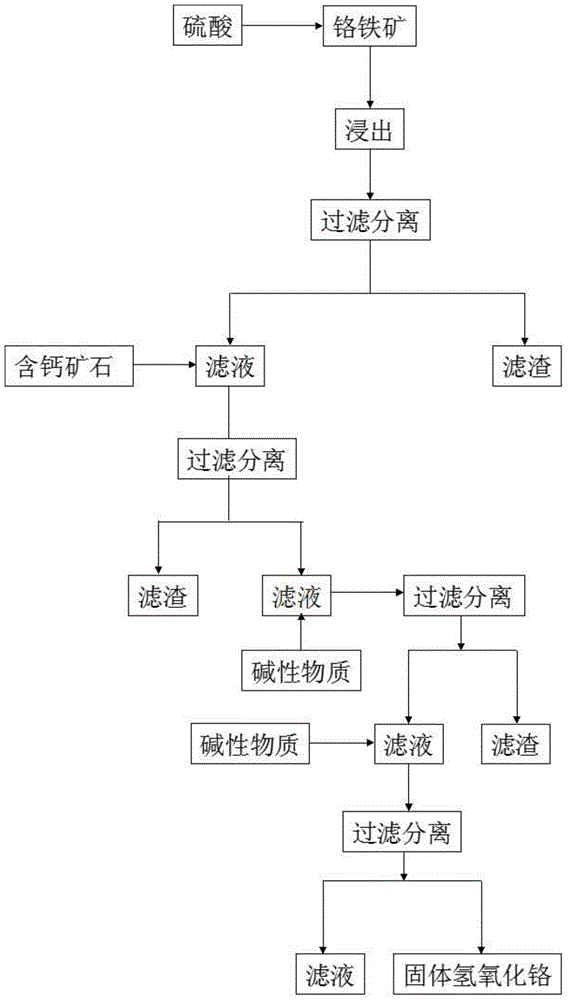
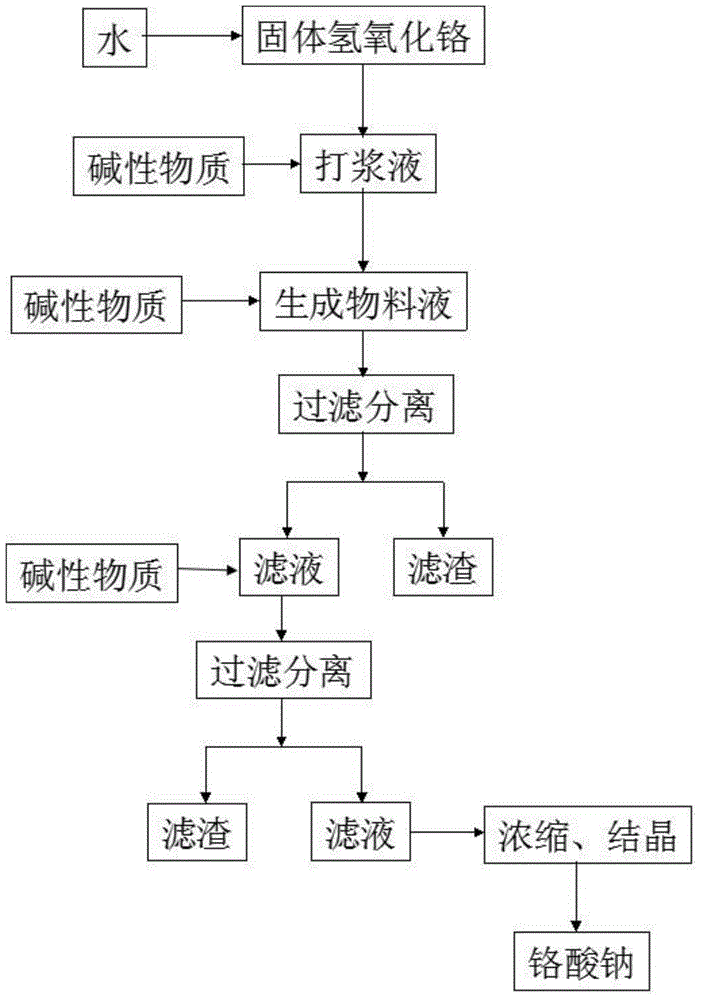
[0054] A method for preparing sodium chromate by acid solution oxidation of chromite, comprising the following steps:
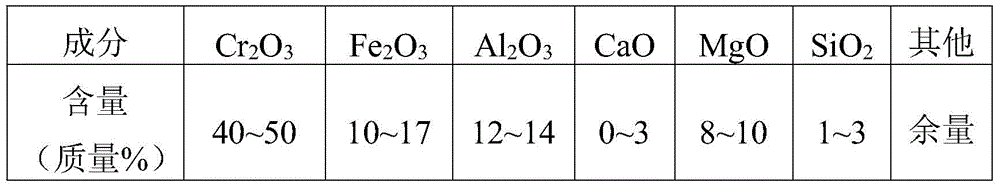
[0055] Step 1: Grind the chromite into mineral powder with a particle size of 200 meshes, then add the mineral powder into the reaction kettle and add 20% sulfuric acid with a mass concentration of 20%. Stir at a high speed and add the oxidant sodium vitriol, the amount of sodium vitriol added is 10% of the mass of the mineral powder, the heating reaction temperature is 50 ° C, and the reaction pressure is controlled to be 1.0MPa and the reaction time is 100min;
[0056] The main chemical reactions in the above process are as follows:
[0057] Fe(CrO 2 ) 2 +4H 2 SO 4 →FeSO 4 +Cr 2 (SO 4 ) 3 +4H 2 O
[0058] (Mg, Fe)Cr 2 O 4 +5H 2 SO 4 →FeSO 4 +Cr 2 (SO 4 ) 3 +MgSO 4 +5H 2 O
[0059] MgCr 2 O 4 +4H 2 SO 4 →MgSO 4 +Cr 2 (SO 4 ) 3 +4H 2 O
[0060] (Mg, Fe) 7 Si 8 O 22 (OH) 2 +7H 2 SO 4 →7FeSiO 3 ↓+7MgSO 4 ↓+SiO 2 ↓+8H 2 O...
Embodiment 2
[0066] A method for preparing sodium chromate by acid solution oxidation of chromite, comprising the following steps:
[0067]Step 1: Grind the chromite into mineral powder with a particle size of 200 meshes, then add the mineral powder into the reaction kettle and add 40% sulfuric acid with a mass concentration of 0.7 times the mass of the mineral powder. Stir at a high speed and add oxidant oxygen, the amount of oxygen added is 20% of the mass of the mineral powder, the heating reaction temperature is 140 ° C, and the reaction pressure is controlled to be 2.0 MPa and the reaction time is 150 min;
[0068] The main chemical reactions in the above process are as follows:
[0069] Fe(CrO 2 ) 2 +4H 2 SO 4 →FeSO 4 +Cr 2 (SO 4 ) 3 +4H 2 O
[0070] (Mg, Fe)Cr 2 O 4 +5H 2 SO 4 →FeSO 4 +Cr 2 (SO 4 ) 3 +MgSO 4 +5H 2 O
[0071] MgCr 2 O 4 +4H 2 SO 4 →MgSO 4 +Cr 2 (SO 4 ) 3 +4H 2 O
[0072] (Mg, Fe) 7 Si 8 O 22 (OH) 2 +7H 2 SO 4 →7FeSiO 3 ↓+7MgSO 4...
Embodiment 3
[0078] A method for preparing sodium chromate by acid solution oxidation of chromite, comprising the following steps:
[0079] Step 1. Grind the chromite into mineral powder with a particle size of 200 meshes, then add the mineral powder into the reactor and add 50% sulfuric acid with a mass concentration of 50%. The amount of sulfuric acid added is 0.6 times the mass of the mineral powder. Stir at a high speed and add oxidant oxygen, the amount of oxygen added is 30% of the mass of the mineral powder, the heating reaction temperature is 200 ° C, and the reaction pressure is controlled to be 4.0 MPa and the reaction time is 200 min;
[0080] The main chemical reactions in the above process are as follows:
[0081] Fe(CrO 2 ) 2 +4H 2 SO 4 →FeSO 4 +Cr 2 (SO 4 ) 3 +4H 2 O
[0082] (Mg, Fe)Cr 2 O 4 +5H 2 SO 4 →FeSO 4 +Cr 2 (SO 4 ) 3 +MgSO 4 +5H 2 O
[0083] MgCr 2 O 4 +4H 2 SO 4 →MgSO 4 +Cr 2 (SO 4 ) 3 +4H 2 O
[0084] (Mg, Fe) 7 Si 8 O 22 (OH) 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com