Quantitative assay method of PEG-modified medicine in biological sample
A measurement method and technology of biological samples, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as non-unique values, and achieve the effects of strong specificity, wide application range, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for quantitative determination of PEGylated drugs in biological samples, which is determined by liquid chromatography-time-of-flight mass spectrometry, and the determination steps include:
[0035] A. Establish a standard curve for free drug determination and a standard curve for PEGylated drug determination,
[0036] B. Use liquid chromatography-time-of-flight mass spectrometry to measure the sample to be tested, and calculate the concentration of free drug and the concentration of PEGylated drug in the sample to be tested by the standard curve obtained in step A;
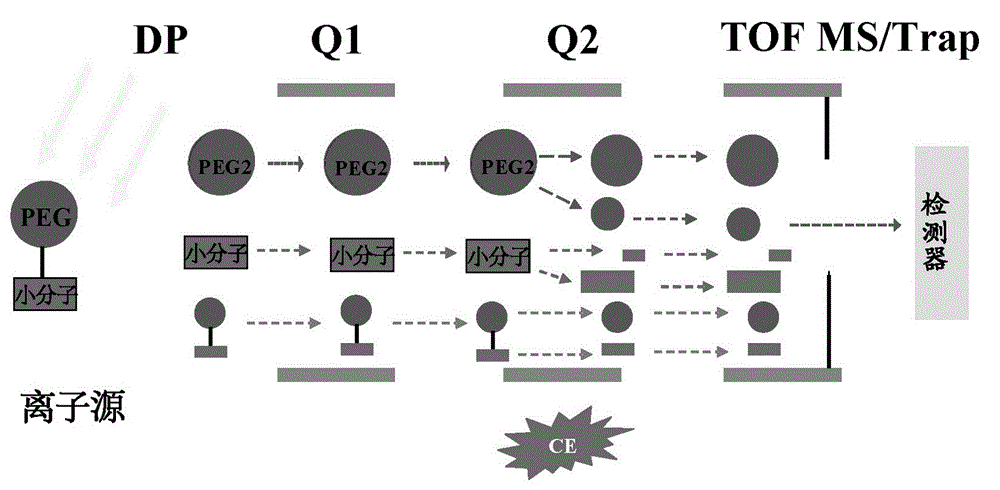
[0037] The chromatographic conditions and mass spectrometry conditions of steps A and B are the same, wherein the mass spectrometry conditions are based on triple tandem mass spectrometry technology, and the setting of the mass spectrometry part of steps A and B is: the voltage in the first mass analyzer Q1 is set to RF (radio frequency voltage) only, no parent ion is selected, all charged particle...
Embodiment 2
[0050] On the basis of Example 1, the PEGylated doxorubicin in plasma was specifically determined.
[0051] A quantitative assay method for PEGylated doxorubicin in a biological sample,
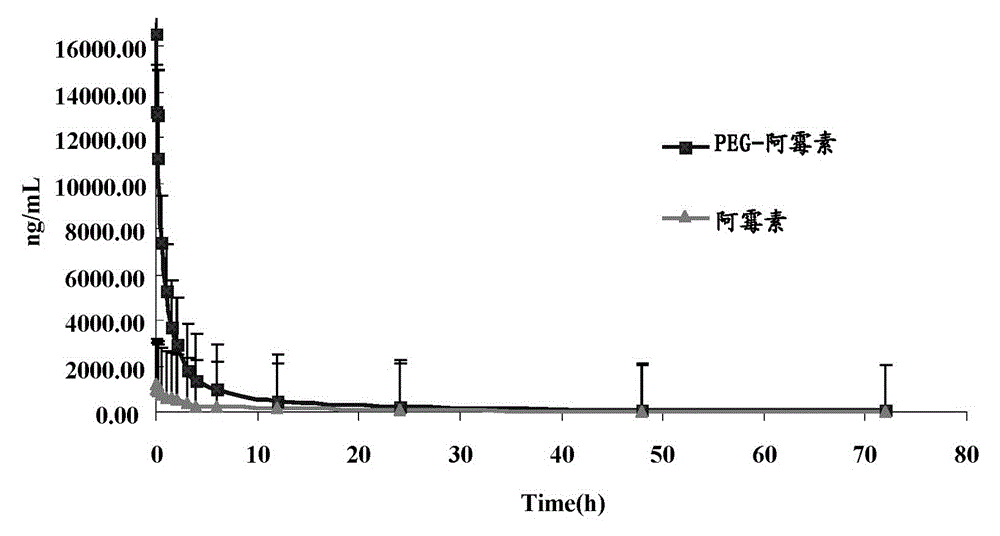
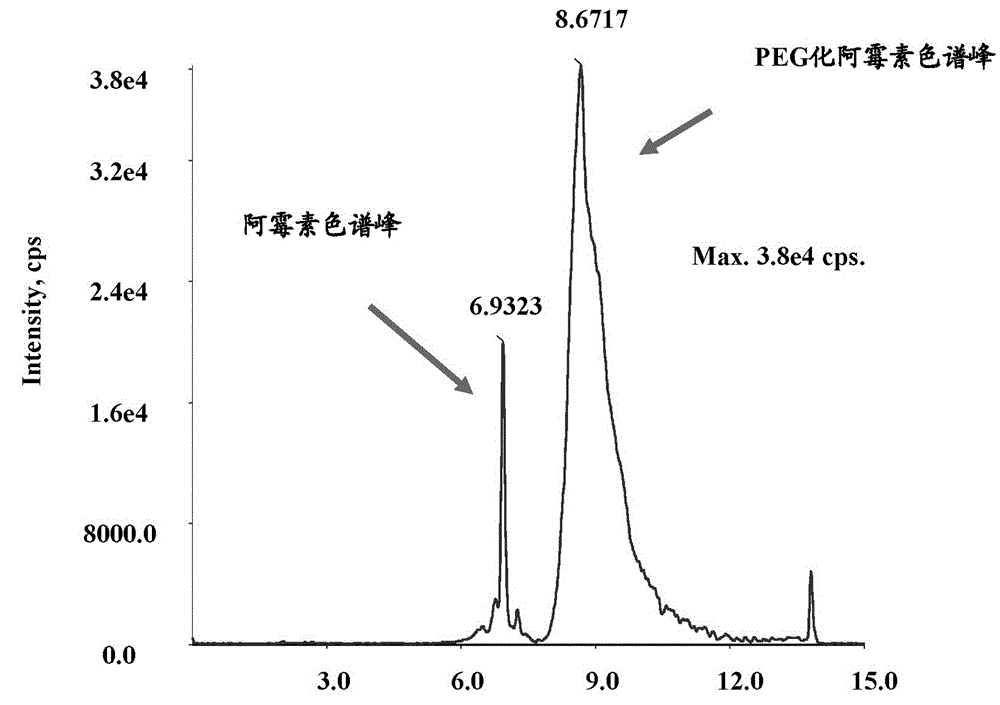
[0052] PEGylated doxorubicin with a drug loading equivalent to 1 mg of doxorubicin was dissolved in 1 mL of normal saline and administered to rats through the tail vein. 2, 3, 4, 6, 12, 24, 48, 72h. Determine the content of free doxorubicin and PEGylated doxorubicin in plasma after rat tail vein administration of PEGylated doxorubicin, the plasma drug concentration time curve is shown in figure 2 .
[0053] Measured by liquid chromatography-time-of-flight mass spectrometry, the determination steps include:
[0054] A. preparation of doxorubicin concentration determination standard curve and PEGylated doxorubicin concentration determination standard curve;
[0055] 1) Dilute doxorubicin and PEGylated doxorubicin stock solution to 0.05, 0.1, 0.3, 1.0, 3.0, 10.0, 30.0 μg / mL with acetonitr...
Embodiment 3
[0071] On the basis of Example 1, PEGylated gemcitabine in plasma was specifically determined.
[0072] A quantitative assay method for PEGylated gemcitabine in a biological sample,
[0073] PEGylated gemcitabine with a drug loading equivalent to 4 mg of gemcitabine was dissolved in 1 mL of normal saline and administered to rat tail veins. The blood collection time points were as follows: 0, 0.083h, 0.25h, 0.75h, 1h, 1.5h, 2h, 4h , 6 h, 9 h, 24 h, 36 h, take 0.5 mL of blood from the orbital vein of the rat, put the plasma in a pre-cooled heparinized EP tube, centrifuge at 4°C (15000 rpm, 5 min), separate the plasma, and take all Supernatant, the supernatant was stored in a -20°C refrigerator for testing. Determination of the content of free gemcitabine and PEGylated gemcitabine in the plasma after rat tail vein administration of PEGylated gemcitabine, the plasma drug concentration time curve is shown in Figure 4 .
[0074] Measured by liquid chromatography-time-of-flight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com