Low-serum protein-free culture medium applicable to Vero cell growth and preparation method thereof
A protein-free medium and low-serum technology, applied in the field of low-serum protein-free medium and its preparation, can solve the problems of standardization and serialization limitations, unclear chemical composition, expensive serum, etc., to achieve easy pollution and benefit The effect of separation and chemical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
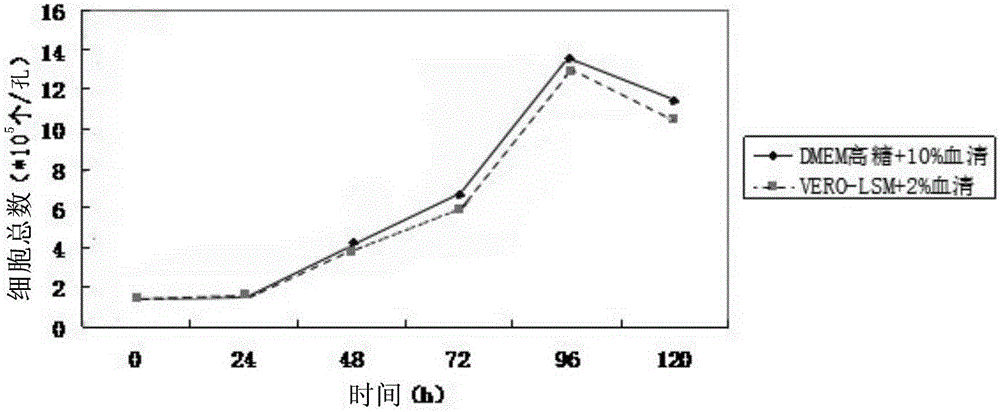
[0041] This example is used to prepare a low-serum, protein-free cell culture medium suitable for Vero cell growth.
[0042] 1. The formulation of the culture medium
[0043] Table 1 medium formula
[0044]
[0045]
[0046]
[0047] 2. Preparation method
[0048] (1) Get the dried components in the formula and put them into the ball mill tank respectively, and ball mill for 2 hours to get the culture medium dry powder;
[0049] (2) Dissolve 16.73 g of the medium dry powder prepared in step (1) in 900 ml ultrapure water, then dilute it to 1 L with ultrapure water, and filter and sterilize it with a 0.22 μm filter membrane to obtain suitable Low-serum, protein-free liquid medium grown on Vero cells, refrigerated at 6°C for later use.
Embodiment 2
[0051] This example is used to prepare a low-serum, protein-free cell culture medium suitable for Vero cell growth.
[0052] 1. The formulation of the culture medium
[0053] Table 2 medium formula
[0054]
[0055]
[0056]
[0057] 2. Preparation method
[0058] (1) Get the dried components in the formula and put them into the ball mill tank respectively, and ball mill for 1.5 hours to get the culture medium dry powder;
[0059] (2) Dissolve the dry medium powder prepared in step (1) in 900ml ultrapure water, then dilute it to 1L with ultrapure water, and filter and sterilize it with a 0.22 μm filter membrane to obtain a Vero Low-serum protein-free liquid medium for cell growth, refrigerated at 8°C for later use.
Embodiment 3
[0061] This example is used to prepare a low-serum, protein-free cell culture medium suitable for Vero cell growth.
[0062] 1. The formulation of the culture medium
[0063] Table 3 medium formula
[0064]
[0065]
[0066]
[0067] 2. Preparation method
[0068] (1) Get the dried components in the formula and put them into the ball mill tank respectively, and ball mill for 3 hours to get the culture medium dry powder;
[0069] (2) Dissolve the dry medium powder prepared in step (1) in 900ml ultrapure water, then dilute it to 1L with ultrapure water, and filter and sterilize it with a 0.22 μm filter membrane to obtain a Vero Low-serum protein-free liquid medium for cell growth, refrigerated at 4°C for later use.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap