Catalyst for compounding fluorine-containing halohydrocarbon and preparation method of catalyst
A technology of catalysts and halogenated hydrocarbons, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as complex processes, high energy consumption, and complicated process flows, and achieve mild preparation conditions, The effect of simple operation and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Preparation of large specific surface area, mesoporous aluminum fluoride
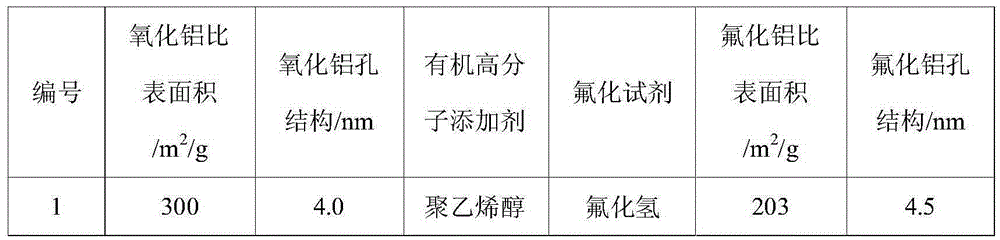
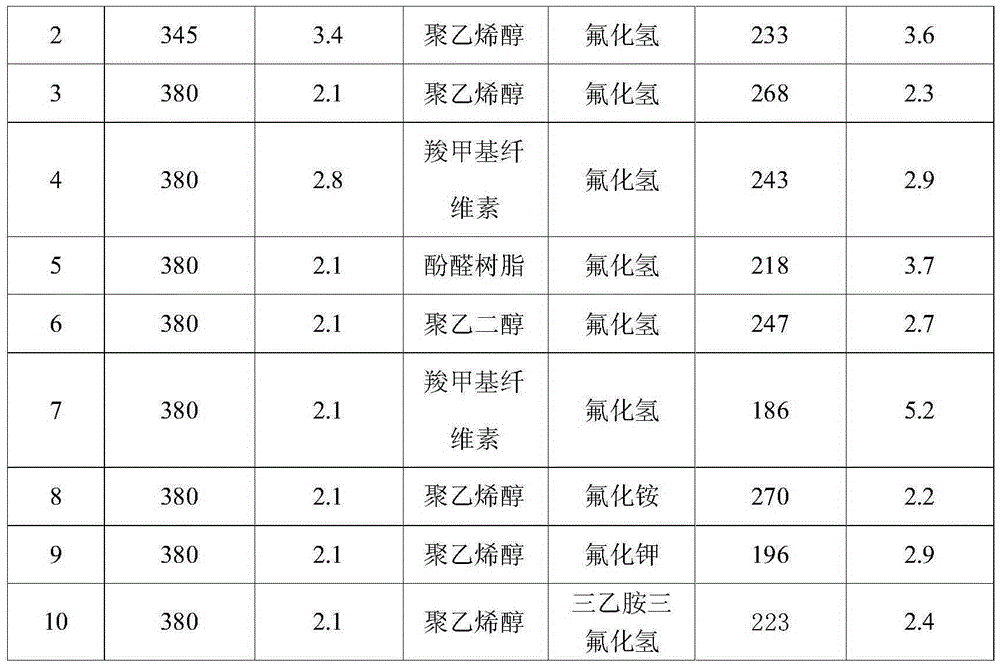
[0053] Dissolve 15.0g of a mixture of large specific surface area, mesoporous alumina, and 15.0g of organic polymer additives in 100mL of water, and reflux the suspension at 60°C for 12 hours, then slowly drop the fluorinated reagent aqueous solution under stirring. Add it to the above suspension, add it dropwise for 2 hours, control the fluorination temperature at 90°C, stir for 6 hours after the dropwise addition, and then age it statically at 50°C for 12 hours, then wash, filter, and dry to obtain a large ratio Surface area, mesoporous structure of aluminum fluoride. The specific surface area and pore size distribution of aluminum fluoride prepared under different alumina specific surface areas, pore structures, organic polymer additives, and fluorinating reagents are shown in Table 1.
[0054] The physical and chemical property results of aluminum fluoride in Example 1 of table 1 ...
Embodiment 2
[0057] Example 2: Preparation of large specific surface area, mesoporous zirconium fluoride
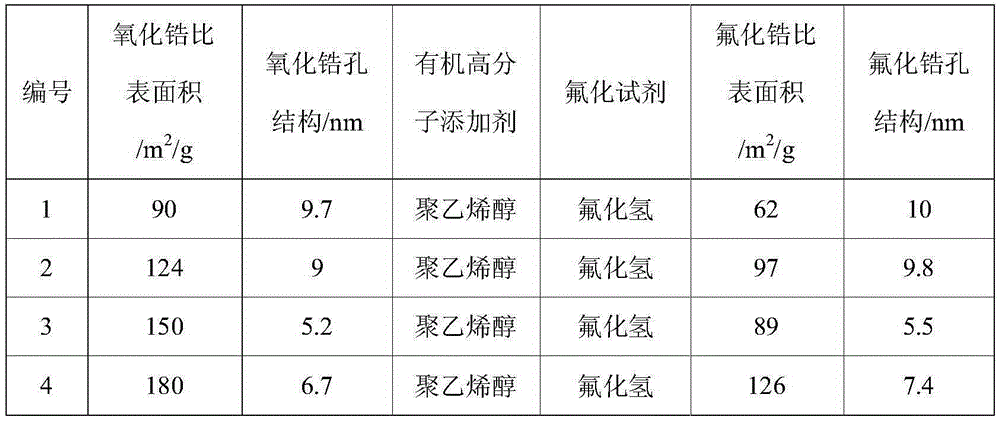
[0058] Dissolve 15.0g of a mixture of large specific surface area, mesoporous alumina, and 30.0g of organic polymer additives in 100mL of water, and reflux the suspension at 80°C for 12 hours, then slowly drop the fluorinated reagent aqueous solution under stirring. Add it to the above suspension, add it dropwise for 4 hours, control the fluorination temperature at 60°C, stir for 8 hours after the dropwise addition, and then age it statically at 50°C for 12 hours, then wash, filter, and dry to obtain a large ratio Surface area, mesoporous structure of aluminum fluoride. The specific surface area and pore size distribution of zirconium fluoride prepared under different zirconia specific surface areas, pore structures, organic polymer additives, and fluorinating reagents are shown in Table 2.
[0059] Zirconium fluoride physicochemical property result of table 2 embodiment 2
[0060] ...
Embodiment 3
[0062] Embodiment 3: Preparation of large specific surface area, mesoporous magnesium fluoride
[0063] Dissolve 15.0g of a mixture of magnesium oxide with large specific surface area, mesoporous structure, and 45.0g of organic polymer additives in 100mL of water, and reflux the suspension at 30°C for 12 hours, then slowly drop the fluorinated reagent aqueous solution under stirring. Add it to the above suspension, add it dropwise for 3 hours, control the fluorination temperature at 30°C, stir for 8 hours after the dropwise addition, and then age it statically at 50°C for 12 hours, then wash, filter, and dry to obtain a large ratio Surface area, mesoporous structure of aluminum fluoride. The specific surface area and pore size distribution of magnesium fluoride prepared under different magnesium oxide specific surface areas, pore structures, organic polymer additives, and fluorinating reagents are shown in Table 3.
[0064] The magnesium fluoride physicochemical property resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com