An expression vector, construction method and application of light-regulated T cell activation
An expression vector, light regulation technology, applied in biochemical equipment and methods, vectors, applications, etc., to achieve the effects of time and space controllability and fast light regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
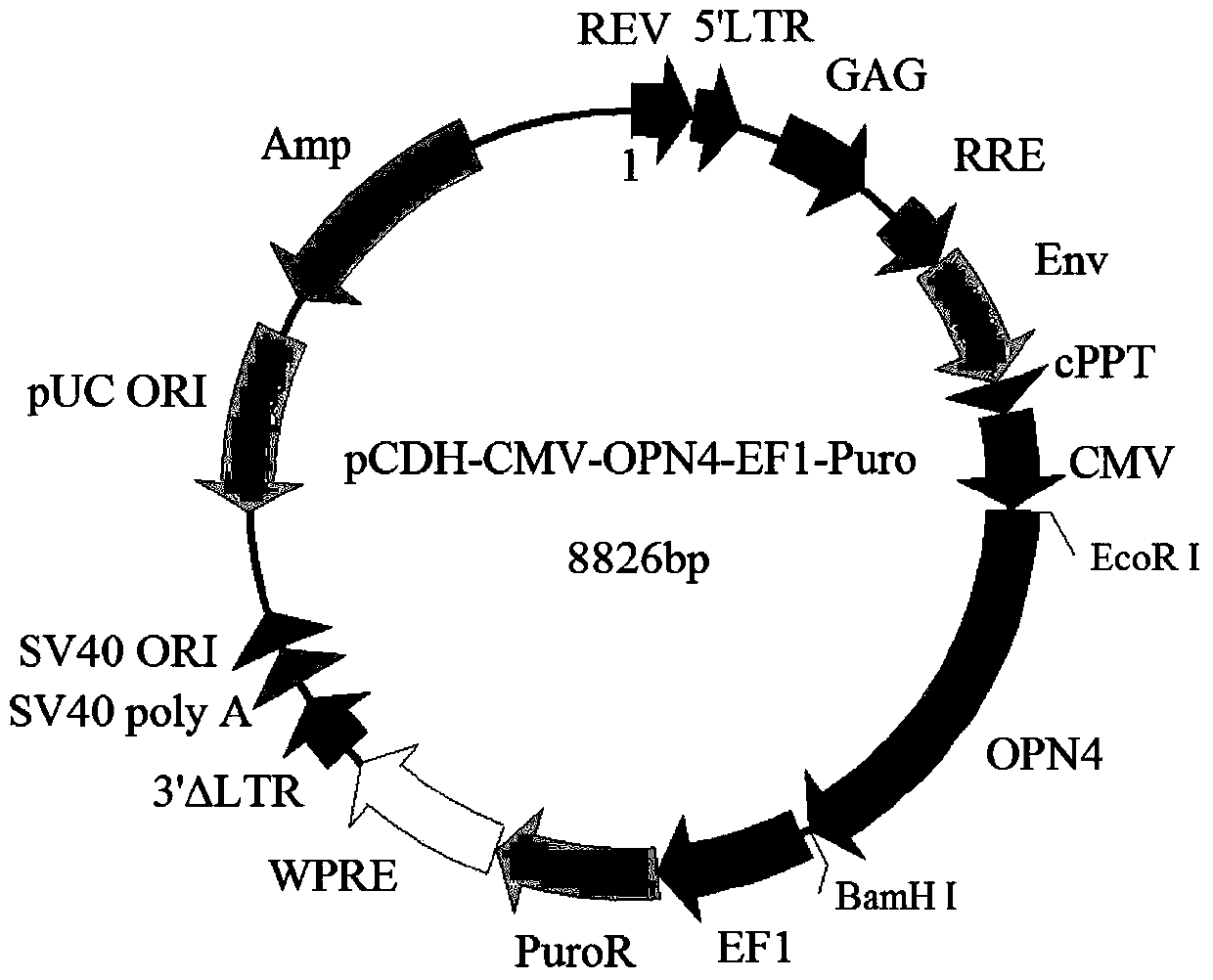
[0035] Example 1: Construction of OPN4 lentiviral expression vector
[0036] Entrusted Suzhou Jinweizhi Biotechnology Co., Ltd. to perform sequence synthesis (SEQ ID No.1 sequence), the synthesized sequence was added with 5'EcoRI and 3'BamHI restriction sites, and cloned into the pUC57 vector to obtain the recombinant vector pUC57-OPN4.
[0037] The synthetic recombinant vector was digested with EcoRI and BamHI to recover and purify the OPN4 gene sequence fragment, and the pCDH-CMV-MCS-EF1-Puro vector plasmid (purchased from Shanghai Jiran Biotechnology Co., Ltd.) was also digested with double enzymes to recover and purify the linear vector .
[0038] The linear vector and the OPN4 gene fragment were ligated at a ratio of 1:5 with T4 DNA ligase (Treasure Bioengineering (Dalian) Co., Ltd.) at 16°C, and the ligated product was transformed into competent E. 100 μg / ml) LB immobilized medium plate (upper, cultivate overnight at 37°C, pick a single colony in LB liquid medium (recip...
Embodiment 2
[0039] Example 2: Lentiviral packaging of OPN4 plasmid
[0040]HEK293T cells were inoculated in 10cm cell culture dishes with DMEM complete medium (DMEM medium containing 10% fetal bovine serum, 100U / ml penicillin, 100U / ml streptomycin), and when they grew to 70%-80% confluence, use Lipofectamin3000 transfection reagent transfects OPN4 lentiviral expression plasmid (pCDH-CMV-OPN4-EF1-Puro) and related lentiviral packaging plasmids (PLP-1, PLP-2 and pLP / VSVG), plasmid transfection amount: PLP-1 6.5 μg, PLP-2 2.5 μg, pLP / VSVG 3.5 μg, pCDH-CMV-OPN4-EF1-Puro 10 μg. After culturing for 12 hours, replace the fresh cell culture medium; continue culturing for 24 hours, collect the virus supernatant by centrifugation, filter through a 0.45 μm filter membrane, and store the obtained virus liquid at -80°C for subsequent infection of cells. (Lentiviral packaging plasmids PLP-1, PLP-2 and pLP / VSVG were purchased from Shanghai Jiran Biotechnology Co., Ltd.).
Embodiment 3
[0041] Example 3: Recombinant lentivirus infection of 293T cells or Jurkat T cells
[0042] 293T cells were inoculated into 6-well plates until they reached 60-80% confluence, and 1.5ml of fresh DMEM complete medium and 0.5ml of virus solution were added;
[0043] Jurkat T cells 8 x 10 5 Each was resuspended with 1ml 1640 complete medium (1640 medium containing 10% fetal bovine serum, 100U / ml penicillin, 100U / ml streptomycin), and 1ml virus liquid was added;
[0044] Both cell lines were added Polybrene to a final concentration of 4 μg / ml, mixed gently, and placed in an incubator for 12 hours. The culture medium containing the virus was absorbed, and fresh DMEM complete medium (293T cells) or 1640 complete medium ( Jurkat T cells). After continuing to culture for 48 hours, change to fresh DMEM complete medium or 1640 complete medium containing 3 μg / ml Puromycin to screen for stably transduced cell lines, and obtain 293T cells and Jurkat T cells stably expressing OPN4 after s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com