Dapagliflozin hemihydrate, crystal form of dapagliflozin hemihydrate, preparation method of crystal form of dapagliflozin hemihydrate and pharmaceutical composition
A hemihydrate and composition technology, which is applied in the field of dapagliflozin hemihydrate and its crystal form, can solve the problems of long preparation time, expensive propylene glycol, and inability to maintain the original crystal form, and achieve cheap solvents and easy preparation The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0074] Preparation Example 1 (Preparation of known dapagliflozin amorphous)
[0075] The known dapagliflozin amorphous can be prepared according to the method described in the patent document US6515117B2 or prepared according to the following method.
[0076] Take 40 mg of dapagliflozin oil, add 400 microliters of dichloromethane to dissolve, and spin dry at room temperature to obtain a white foamy dapagliflozin amorphous.
Example Embodiment
[0077] Preparation Example 2 (Preparation of known dapagliflozin amorphous)
[0078] Take 500 mg of Dapagliflozin oil, add 5 ml of dichloromethane to dissolve, and spin to dry at room temperature to obtain part of the oil and part of the foamy solid to mix. Add 5 ml of n-heptane, stir at room temperature for 4 hours, filter under reduced pressure, and vacuum dry the filter cake at room temperature for 24 hours to obtain dapagliflozin amorphous.
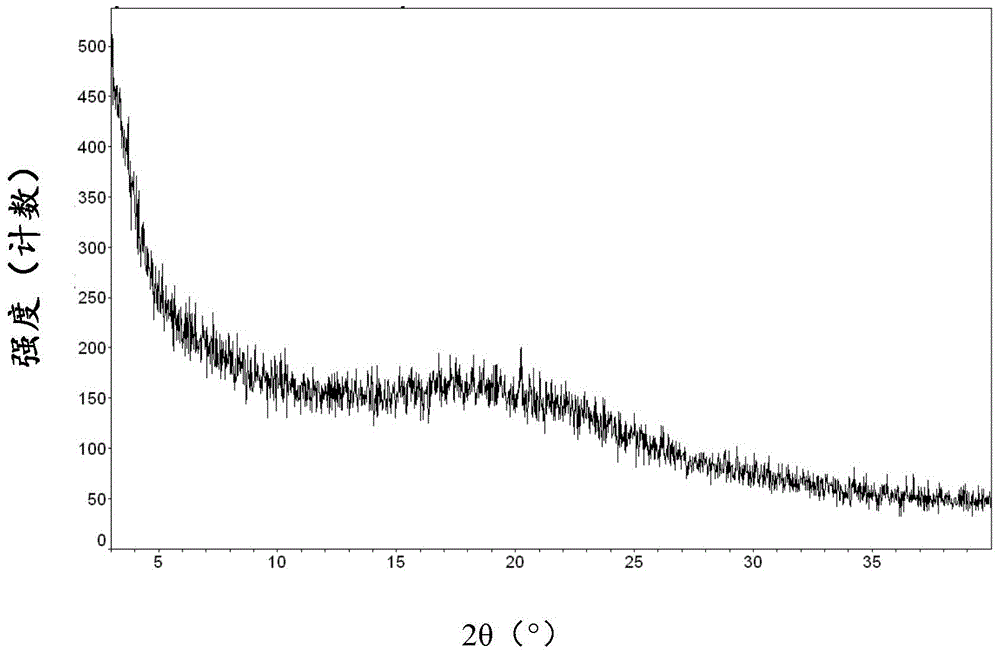
[0079] XRPD diagram see figure 1 , appearing as amorphous.
Example Embodiment
[0080] Preparation Example 3 (Preparation of known dapagliflozin dihydrate crystal form A)
[0081] The known dapagliflozin dihydrate crystal form A can be prepared according to the method described in Example 3 of the patent document CN103958491A or according to the following method.
[0082] The specific preparation method is as follows: take 50 mg of amorphous dapagliflozin prepared in Preparation Example 2, suspend it in 3 ml of water, and heat it to 80°C. To this mixture was added a solution of 20 mg of xylitol in 0.5 ml of water to form an emulsion, which was cooled to 5°C at a rate of 2°C per hour. At this temperature, the mixture was stirred for about 20 hours or until crystals could be detected by optical microscopy. After crystallization, the suspension was filtered and the solid product was dried in air at room temperature at about 50% relative humidity for about 1 hour to yield Dapagliflozin dihydrate Form A.
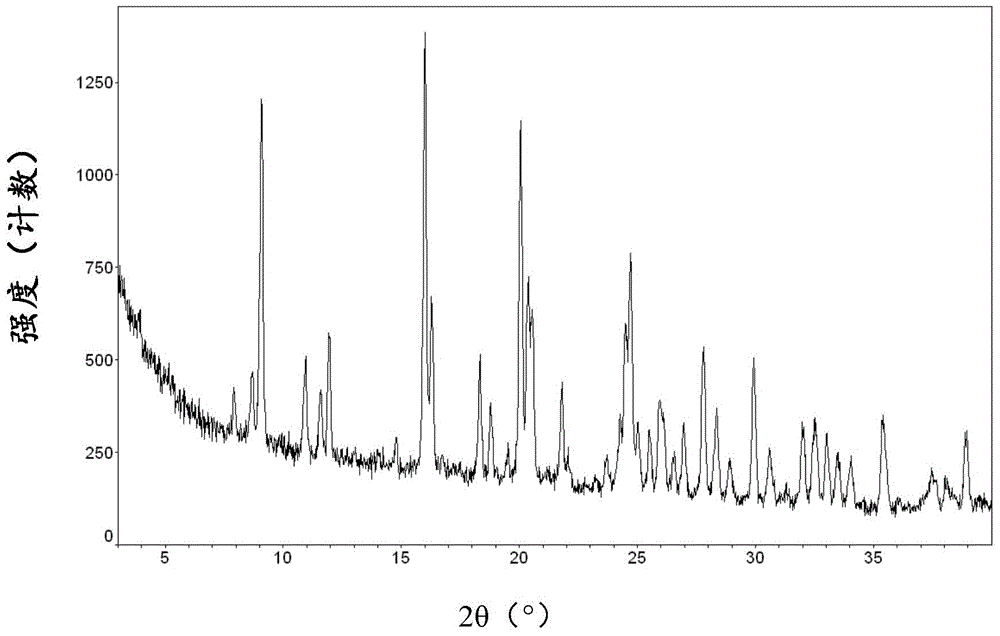
[0083] XRPD diagram see figure 2 , shown to be c...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap