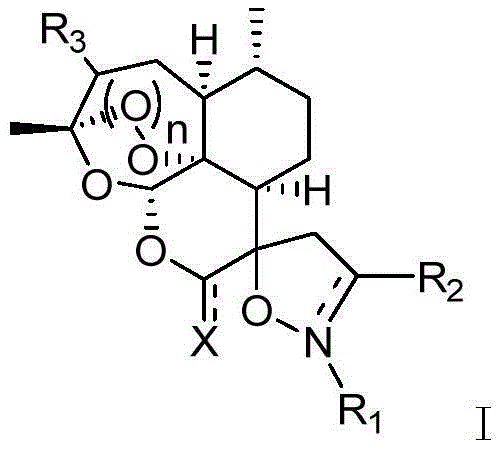
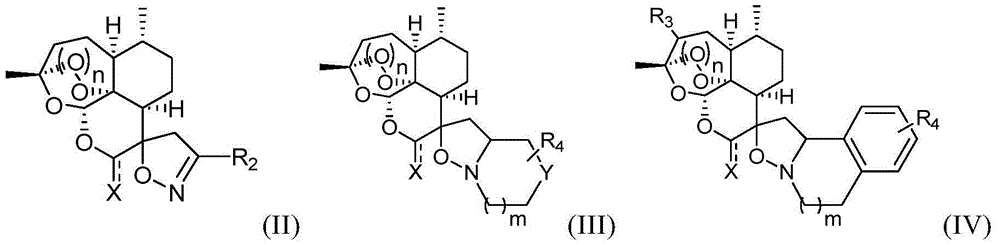
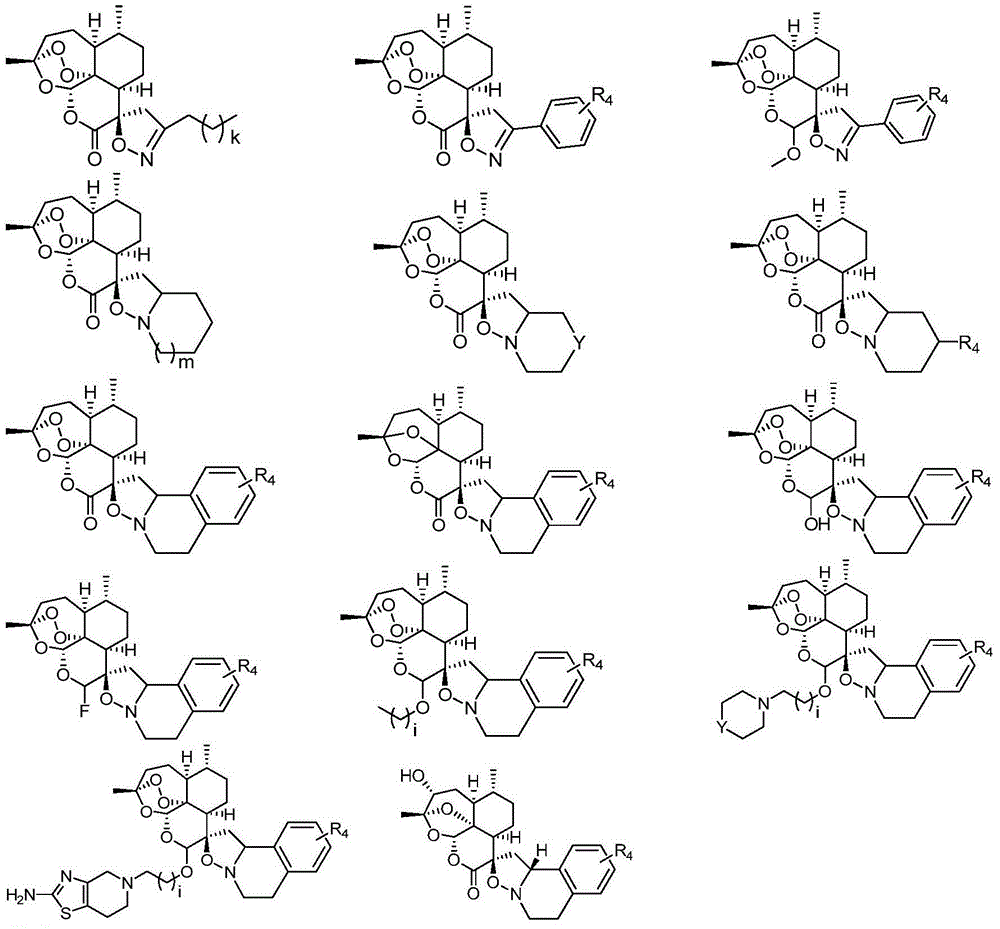
Arteannuim derivate substituted by isoxazoline and isoxazolidine and preparation method and application thereof
An unsubstituted and substituent technology, applied in the preparation of anticancer drugs, field of artemisinin derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Embodiment 1, the synthesis of compound S0
[0158]
[0159] The reaction process is shown in scheme1:
[0160]
[0161] Scheme 1
[0162] The preparation of intermediate 1 refers to J.Med.Chem.1997,40,633-638.
[0163] The preparation of intermediate 2 refers to Bioorganic & Medicinal Chemistry Letters.2003,13,1795–1799.
[0164] Intermediate 1 (56mg, 0.2mmol) was dissolved in 2ml of dry CH2Cl2, Intermediate 2 (33mg, 0.21mmol) was added, cooled to 0°C, triethylamine (24mg, 0.22mmol) was slowly added dropwise, and reacted at room temperature for about 12 hours , until the disappearance of intermediate 1 detected by TLC, the reaction was stopped. Add 15 ml of water, extract with CH2Cl2 three times, and the organic layer is washed with water and saturated brine successively, and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure and separated by column chromatography to obtain 73 mg of the target compound as a white solid. ...
Embodiment 2
[0167] Embodiment 2, the synthesis of compound S1
[0168]
[0169] 1 H NMR (300MHz, Chloroform-d) δ7.58(d, J=8.0Hz, 1H), 6.87(d, J=8.1Hz, 1H), 5.97(s, 1H), 3.91(d, J=17.7Hz ,1H),3.81(s,3H),3.61(d,J=17.7Hz,1H),2.41-2.29(m,2H),2.21-2.15(m,1H),2.10–1.97(m,2H), 1.82-1.77(m,1H),1.54–1.28(m,7H),1.12–0.97(m,4H).
Embodiment 3
[0170] Embodiment 3, the synthesis of compound S2
[0171]
[0172] 1 H NMR (300MHz, Chloroform-d) δ7.70–7.66(m,2H),7.40-7.38(m,3H),6.01(s,1H),3.97(d,J=17.9Hz,1H),3.67( d,J=17.9Hz,1H),2.50–2.32(m,2H),2.26-2.20(dd,J=13.2,4.1Hz,1H),2.14–2.20(m,2H),1.86-1.80(m, 1H),1.56–1.31(m,7H),1.15–1.00(m,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com