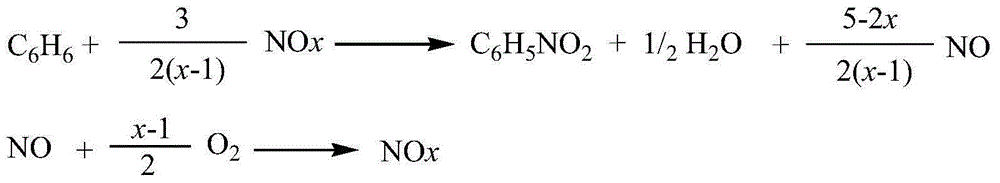Method for preparing nitrobenzene from benzene
A technology of nitrobenzene and nitration reaction, applied in the preparation of nitro compounds, organic chemistry, etc., can solve the problems of high equipment requirements, high energy consumption for product separation, and large amount of nitric acid, so as to simplify the process and equipment and improve resources Utilization rate, effect of product cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: take by weighing 2.5g benzene and 7.37gNO 2 (the molar ratio is 1:5) is placed in a 100ml sealable tank reactor (i.e. a high-pressure reactor made of stainless steel), the reaction pressure is 0.15 MPa, after reacting at 90°C for 6h, cooling, the reaction product is carried out The internal standard analysis by gas chromatography showed that the conversion rate of benzene was 37.1%, and the selectivity of nitrobenzene was 90.4%.
Embodiment 2
[0057] Embodiment 2: The reaction steps are the same as in Example 1, the difference is that in the reactor, add 0.2g HPW / MCM-41 catalyst (prepared by impregnation method, the loading capacity of phosphotungstic acid HPW is 30%, and the activation roasting temperature is 400°C, the activation time is 3h), the conversion rate of benzene is 58.7%, and the selectivity of mononitrobenzene is 98.2%.
Embodiment 3
[0058] Embodiment 3: The reaction steps are the same as in Example 1, the difference is that the nitrating agent is N 2 o 3 , the conversion rate of benzene obtained was 27.5%, and the selectivity of nitrobenzene was 89.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com