Hyperbranched CTP-TPY (Cyclotriphosphazene Terpyridyl), preparation method and recognition method of metal ions
A technology of terpyridine and tripolymer phosphazene, which is applied in chemical instruments and methods, organic chemistry, fluorescence/phosphorescence, etc., can solve the problems of weak recognition ability and single recognition site, and achieve rapid response, high selective recognition ability, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0034] Example 1: Preparation of hexa(4-carbaldehyde phenoxy) cyclophosphazene (CTP-CHO):
[0035] In a 250ml single-necked flask, 100ml of tetrahydrofuran, 2.50g (7.19mmol) of hexachlorocyclotriphosphazene, 6.30g (51.59mmol) of p-hydroxybenzaldehyde, 11.38g (82.30mmol) of potassium carbonate, and 11.38g (82.30mmol) of potassium carbonate were added to the flask with heating and stirring at 66 degrees. Condensate and reflux for 48h.
[0036] After the reaction is complete, change to a distillation device to distill off the solvent tetrahydrofuran, then add the product to 1000ml of distilled water, stir for half an hour, get a white precipitate at rest, filter with suction, wash with distilled water three times, and place it in a vacuum drying cabinet to dry at 60 degrees. The product was recrystallized from ethyl acetate to obtain the product hexa(4-formaldehyde phenoxy) cyclophosphazene (CTP-CHO). The reaction equation is as follows:
[0037]
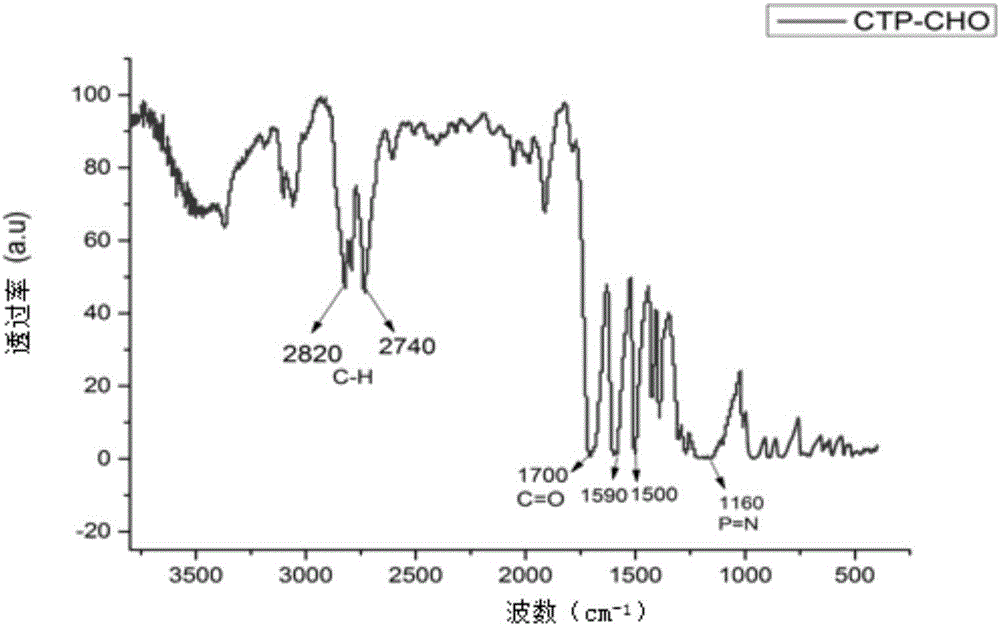
[0038] As attached figure 1 It can b...
Example Embodiment
[0039] Example 2: Preparation of hyperbranched polyphosphazene terpyridine CTP-TPY:
[0040] Add 4.04 g (4.14 mmol) of hexa(4-formaldehyde phenoxy) cyclophosphazene, 7.18 g (55.68 mmol) of 2-acetylpyridine, 120 ml of absolute ethanol and 200 ml of tetrahydrofuran to a 500 ml three-necked flask to completely dissolve Then a light yellow solution was obtained, then 3.894g (55.68mmol) of potassium hydroxide KOH and 90ml of ammonia were added, and stirred at room temperature. The solution became light brown. After 12 hours of reaction, the reaction was stopped and the tetrahydrofuran was removed by rotary evaporation with a rotary evaporator to obtain A large amount of milky yellow precipitate was filtered, and then washed 2-3 times with a large amount of absolute ethanol to obtain a milky yellow powder, which was placed in a vacuum drying oven at 60°C and dried overnight. The reaction process is as follows:
[0041]
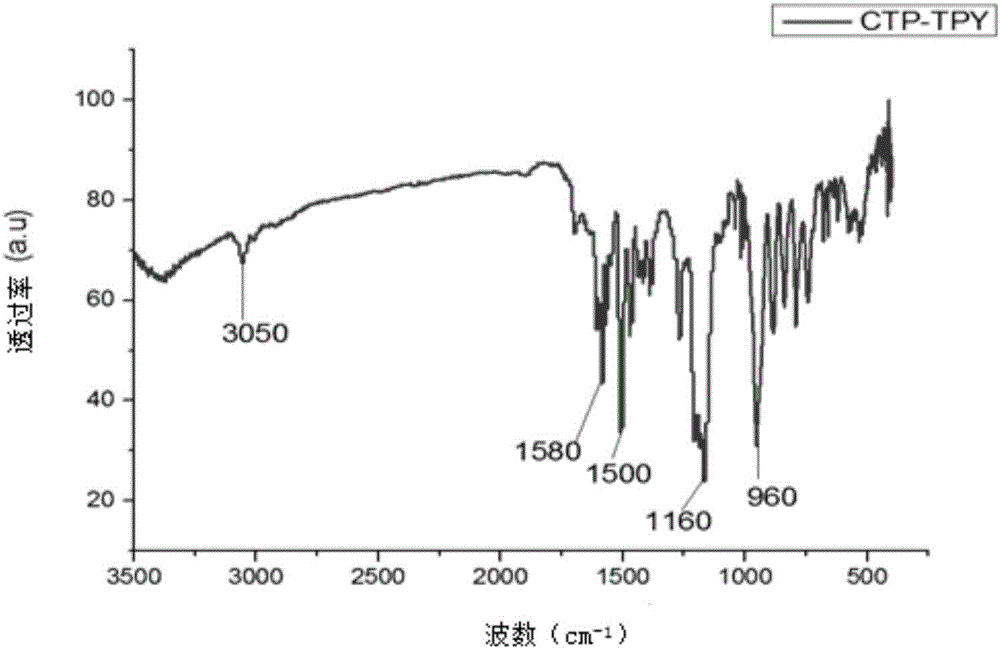
[0042] As attached figure 2 It can be seen from the mid-infrared ...
Example Embodiment
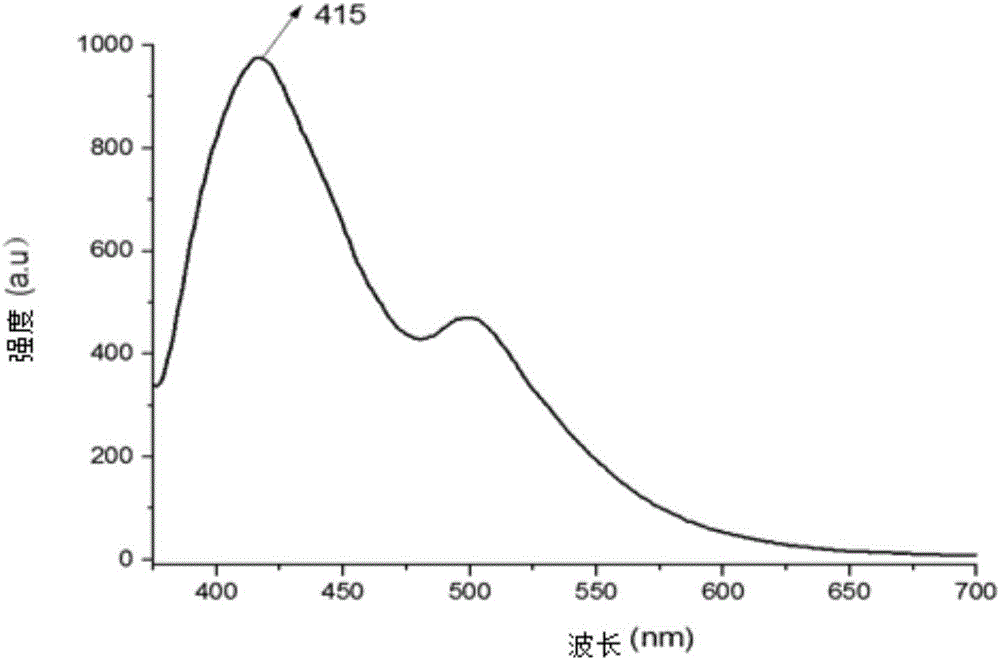
[0045] Example 3: The selective recognition of various metal ions by hyperbranched trimeric phosphazene terpyridine CTP-TPY, the fluorescence titration process is as follows:
[0046] 1. Use a 50ml volumetric flask to prepare supramolecular trimeric phosphazene terpyridine CTP-TPY solution, the solvent is tetrahydrofuran, and the concentration of the solution is 1×10 -4 mol / L.
[0047] 2. Use a 50ml volumetric flask to prepare Zn separately 2+ , Ni 2+ , K + , Mg 2+ , Na + , Fe 3+ , Ca 2+ , Ba 2+ , Pd 2+ , Hg + , Cu 2 + , Li + , Co 2+ , Eu 3+ , Tb 3+ 15 kinds of metal cation ethanol solutions, the concentration of the solution is 1×10 -2 mol / L.
[0048] 3. All fluorescence titration experiments are carried out under the condition of 25±0.5℃, the body (CTP-TPY) used in each titration experiment is 3mL, and the metal cation solution is gradually dripped from 5ul, 10ul, 20ul, 30ul, and 60ul. After each instillation, use a dropper to mix well and then perform the fluorescence test 3 minut...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap