Beta-cyclodextrin-sorbic acid inclusion compound and preparation method thereof
A technology of cyclodextrin and sorbic acid, applied in the field of food additive synthesis, can solve the problems of poor water solubility of sorbic acid, strong odor, easy oxidation and discoloration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Preparation of β-cyclodextrin-sorbic acid inclusion compound
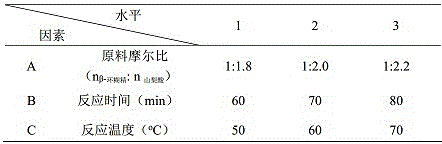
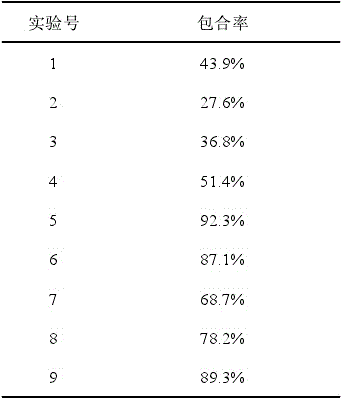
[0015] Dissolve 1.4800 g (0.001 mol) of β-cyclodextrin in 40 mL of deionized water, and 0.2632 g (0.018 mol) of sorbic acid in 2.0 mL of absolute ethanol, 50 o Under ultrasonic conditions, the sorbic acid ethanol solution was slowly added dropwise to the β-cyclodextrin aqueous solution, and the ultrasonic was continued for 60 min. After the reaction was completed, the reaction solution was moved to a cool place, and after 72 h of natural volatilization, crystals were precipitated. Suction filtration, washed several times with a small amount of deionized water and absolute ethanol, at 60 o C under vacuum for 3 h to obtain a white powder clathrate. The inclusion compound contained 0.1154 g of sorbic acid, and the inclusion rate was 43.9%, detected by ultraviolet-visible spectroscopy.
Embodiment 2
[0016] Example 2 Preparation of β-cyclodextrin-sorbic acid inclusion compound
[0017] Dissolve 1.4800 g (0.001 mol) of β-cyclodextrin in 40 mL of deionized water, and 0.2924 g (0.020 mol) of sorbic acid in 2.3 mL of absolute ethanol, 50 o Under ultrasonic conditions, the sorbic acid ethanol solution was slowly added dropwise to the β-cyclodextrin aqueous solution, and the ultrasonic wave was continued for 70 min. After the reaction was completed, the reaction solution was moved to a cool place, and after natural volatilization for 72 h, crystals were precipitated. Suction filtration, washed several times with a small amount of deionized water and absolute ethanol, at 60 o C under vacuum for 3 h to obtain a white powder clathrate. The inclusion compound contained 0.0806 g of sorbic acid, and the inclusion rate was 27.6%, detected by ultraviolet-visible spectroscopy.
Embodiment 3
[0018] Example 3 Preparation of β-cyclodextrin-sorbic acid inclusion compound
[0019] Dissolve 1.4800 g (0.001 mol) of β-cyclodextrin in 40 mL of deionized water, and 0.3217 g (0.022 mol) of sorbic acid in 2.5 mL of absolute ethanol, 50 o Under ultrasonic conditions, the sorbic acid ethanol solution was slowly added dropwise to the β-cyclodextrin aqueous solution, and the ultrasonic wave was continued for 80 min. After the reaction was completed, the reaction solution was moved to a cool place, and after natural volatilization for 72 h, crystals were precipitated. Suction filtration, washed several times with a small amount of deionized water and absolute ethanol, at 60 o C under vacuum for 3 h to obtain a white powder clathrate. The inclusion compound contained 0.1182 g of sorbic acid, and the inclusion rate was 36.8%, detected by ultraviolet-visible spectroscopy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com