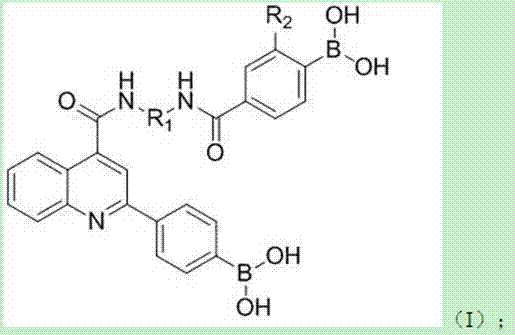
Hypoboric acid derivative based on 2-(4-dyhydroxy borane)pheoylquinoline-4-carboxylic acid and preparation method and application thereof
A technology of dihydroxyborane and phenylquinoline, which is applied in the field of fluorescence recognition, can solve the problems of high price, unfavorable derivatization and carrying out subject research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
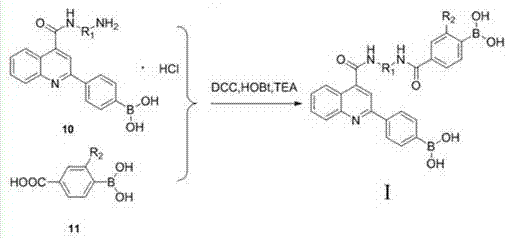
[0066] The present invention also includes a method for preparing diboronic acid derivatives based on 2-(4-dihydroxyborane) phenylquinoline-4-carboxylic acid, comprising the following steps:
[0067] ① Dissolve p-bromoacetophenone and isatin in ethanol, add potassium hydroxide solution to it, stir and reflux for 5-10 hours, distill off the ethanol under reduced pressure, adjust the pH to 2-4 with hydrochloric acid, and precipitate a reddish-brown precipitate , to obtain a filter cake by filtration; the filter cake was washed with water and ethyl acetate successively during filtration, and then the filter cake was recrystallized with a mixture of methanol and glacial acetic acid with a volume ratio of 1:1 to obtain 2-(4-bromophenyl)quinoline -4-carboxylic acid;
[0068] Wherein the molar volume ratio of p-bromoacetophenone, isatin, ethanol and potassium hydroxide solution is 1~1.5mol: 1mol: 1~1.5L: 0.5~1L; the concentration of described potassium hydroxide solution is 30~50 %;...
Embodiment 1
[0119] A diboronic acid derivative based on 2-(4-dihydroxyborane)phenylquinoline-4-carboxylic acid with the structure I 1 Shown:
[0120]
[0121] The preparation method of this compound comprises the following steps:
[0122] ①Dissolve 199g of p-bromoacetophenone and 147g of isatin in 1L of ethanol, add 500ml of potassium hydroxide solution with a concentration of 30%, stir and reflux for 5 hours, remove the ethanol by distillation under reduced pressure, and adjust the pH to 2 with hydrochloric acid , precipitated a reddish-brown precipitate, and filtered to obtain a filter cake; when filtering, the filter cake was washed with 500ml of water and 500ml of ethyl acetate in sequence, and then the filter cake was recrystallized with 500ml of methanol and glacial acetic acid mixture with a volume ratio of 1:1 to obtain 2- (4-bromophenyl)quinoline-4-carboxylic acid;
[0123] ② Take 196g of 2-(4-bromophenyl)quinoline-4-carboxylic acid obtained in step ① into the reaction vesse...
Embodiment 2
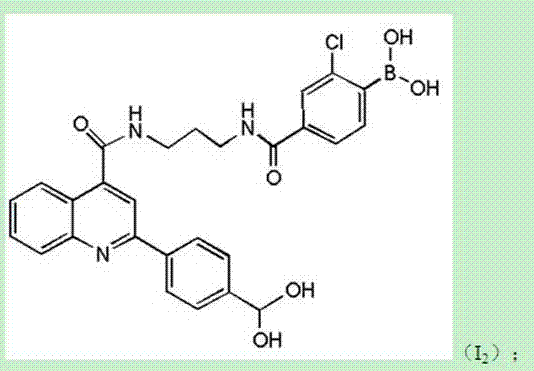
[0134] A diboronic acid derivative based on 2-(4-dihydroxyborane)phenylquinoline-4-carboxylic acid with the structure I 2 Shown:
[0135]
[0136] The preparation method of this compound comprises the following steps:
[0137] ①Dissolve 298.5g of p-bromoacetophenone and 147g of isatin in 1500ml of ethanol, add 1L of 50% potassium hydroxide solution to it, stir and reflux for 10 hours, remove the ethanol by distillation under reduced pressure, and adjust the pH to 4. Precipitate a reddish-brown precipitate, filter to obtain a filter cake; when filtering, the filter cake is washed with 1000ml of water and 1000ml of ethyl acetate in sequence, and then the filter cake is recrystallized with a mixture of methanol and glacial acetic acid with a volume ratio of 1:1 to obtain 2- (4-bromophenyl)quinoline-4-carboxylic acid;
[0138] ② Take 228.9g of the 2-(4-bromophenyl)quinoline-4-carboxylic acid obtained in step ① into the reaction vessel, add 0.7L of thionyl chloride and a drop ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com