drug combination of nep inhibitor and its application
A technology of inhibitors and drugs, applied in the field of biomedicine, can solve problems such as blood reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] components Composition per unit (mg) composition(%) compound 1 calcium salt 25 62.2% microcrystalline cellulose 7 17.4% Low-substituted hydroxypropyl cellulose 5 12.4% Crospovidone 2 5.0% talcum powder 0.4 1.0% colloidal silica 0.2 0.5% Magnesium stearate 0.6 1.5% total 40.2 100%
[0063] The magnesium stearate, colloidal silicon dioxide and microcrystalline cellulose are first sieved through a 30-mesh sieve. The above mixture, active ingredient compound 1 calcium salt polymorph, cross-linked polyvinylpyrrolidone and povidone were then mixed in a hopper mixer for about 120 revolutions. The mixture was pressed using a roller press with a pressure of 30 kN. After pressing, the mixture was ground using a grinder and sieved through a 18 mesh sieve to obtain the final internal phase or granules. Fill the granules into capsules to make capsules.
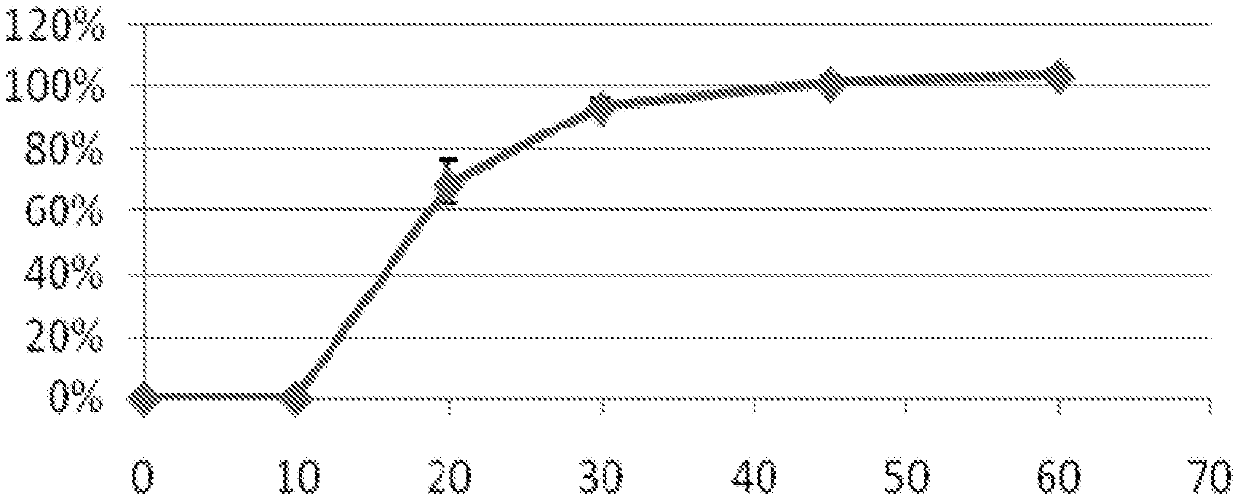
[0064] The dissolution test that gained capsule is car...
Embodiment 2
[0066] components Composition per unit (mg) composition(%) compound 1 calcium salt 123 35.1 Valsartan 98 28 microcrystalline cellulose 61 17.41 Low-substituted hydroxypropyl cellulose 43.5 12.44 Crospovidone 17.5 4.98 colloidal silica 1.75 0.5 Magnesium stearate 5.25 1.5 total weight 350 100
[0067] Firstly, the active ingredient compound 1 calcium salt and valsartan were sieved through a 40-mesh sieve. The microcrystalline cellulose and crospovidone were added to the active ingredient, and the mixture was sieved through a 20-mesh sieve. The mixture was then mixed and rotated about 100 revolutions in a hopper mixer. Then add the low-substituted hydroxypropyl cellulose and colloidal silicon dioxide into the hopper mixer, and make it rotate for 100 revolutions and finally add the magnesium stearate. The powder mixture is then compressed into tablets.
[0068] The tablet that embodiment 2 is prepared is ...
Embodiment 3
[0071]
[0072]
[0073] The magnesium stearate, colloidal silicon dioxide and microcrystalline cellulose are first sieved through a 30-mesh sieve. The above mixture, active ingredients compound 1 and valsartan, crospovidone and povidone were then mixed in a hopper mixer for about 120 revolutions. The mixture was pressed using a roller press with a pressure of 30 kN. After pressing, the mixture was ground using a grinder and sieved through a 18 mesh sieve to obtain the final internal phase or granules. Fill the granules into capsules to make capsules.
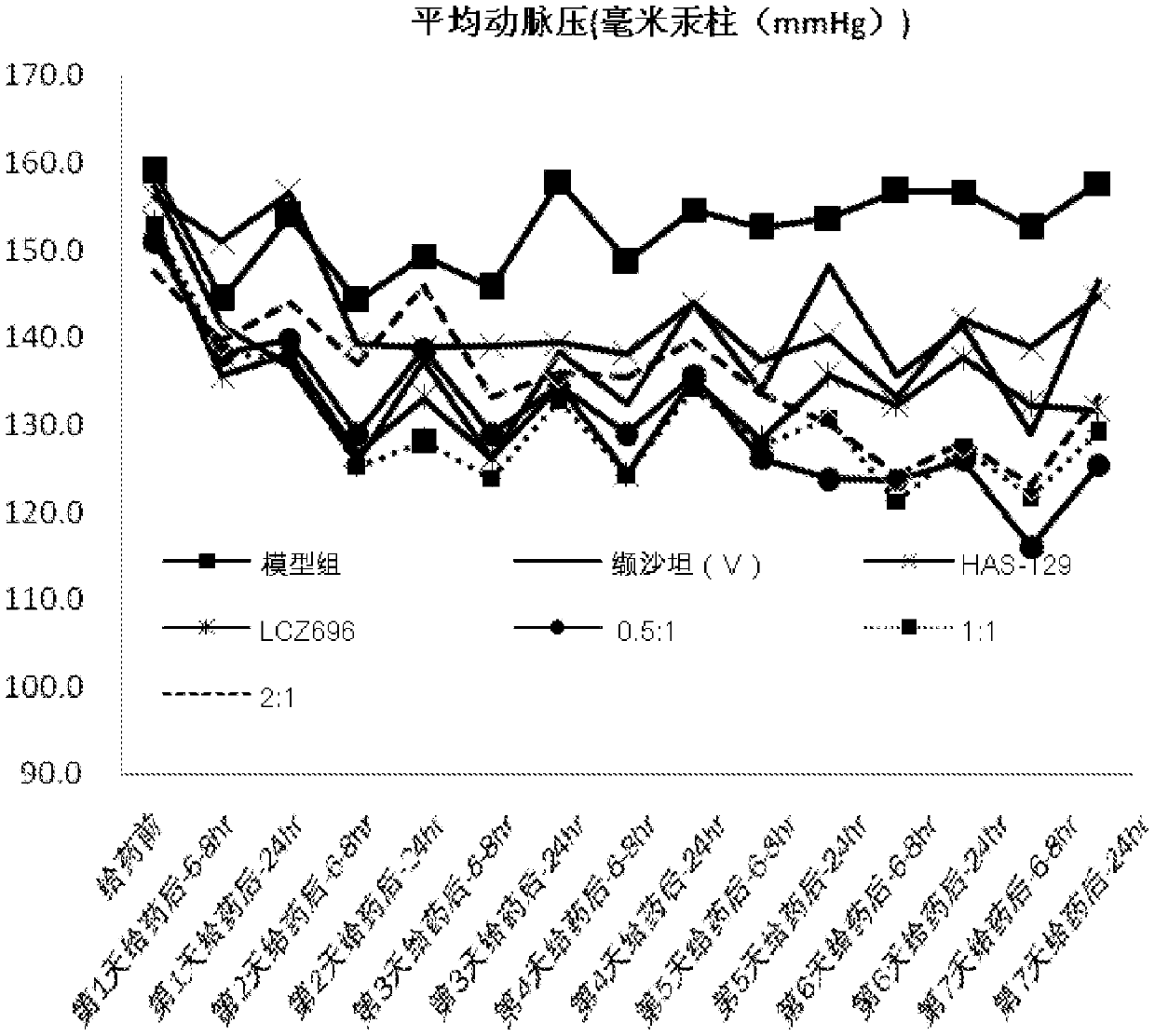
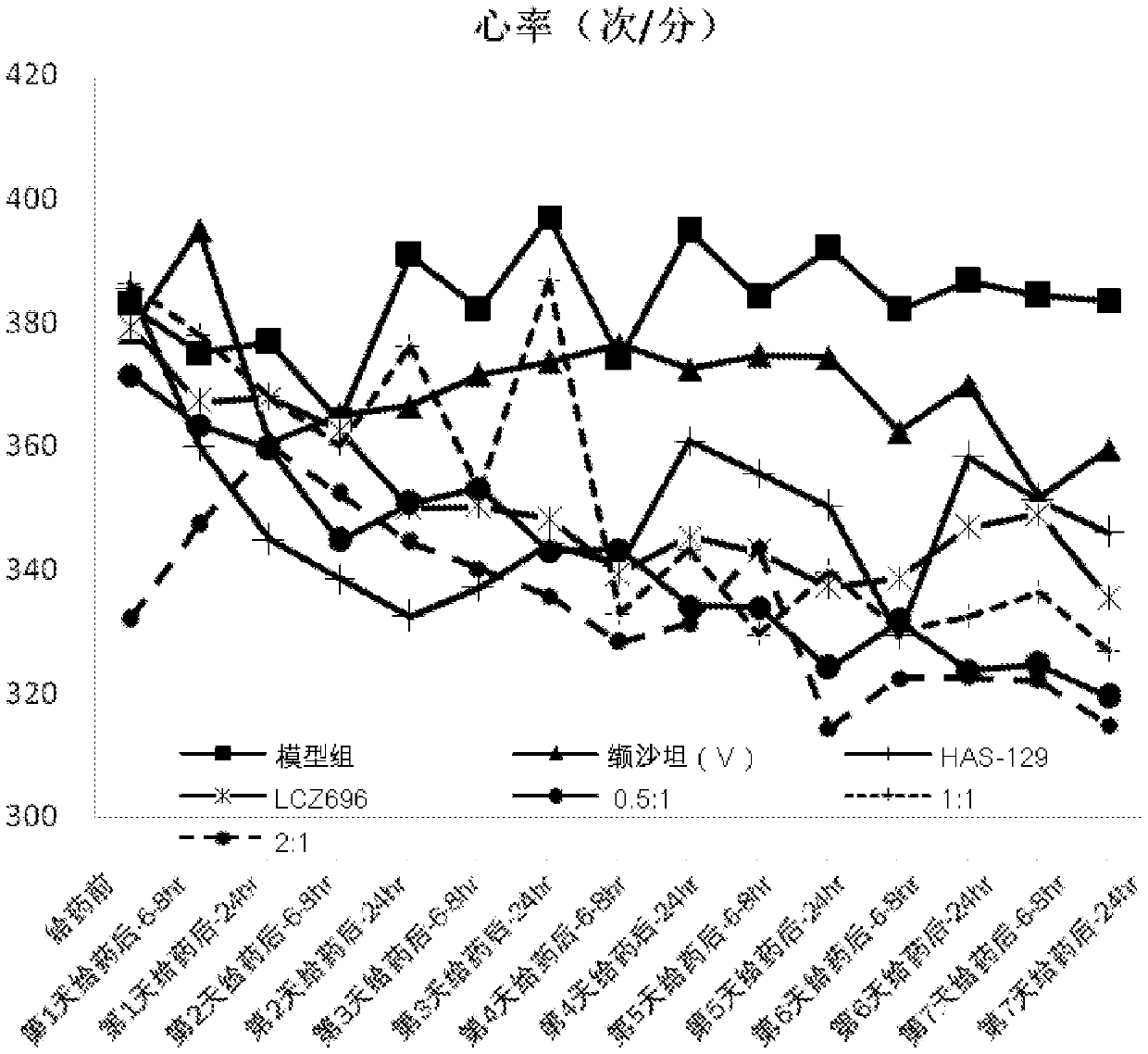
[0074] The capsules prepared in Example 3 are placed under different conditions respectively, and the stability of the preparation is investigated, and the results are as shown in the table:
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com