Acquisition and application of anti-HCV (hepatitis C virus) antibody
A technology of antibodies and viruses, applied in the direction of antiviral agents, antiviral immunoglobulins, applications, etc., can solve problems such as unsuccessful marketing, and achieve the effect of reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0045] 1. Memory B cell sorting strategy
[0046] Flow Cytometry (FCM) is a high-speed, cell-by-cell quantitative analysis and sorting technique that represents a highly effective tool for the selection of high-affinity human monoclonal antibodies. In fact, flow-based sorting steps are very flexible and can be optimized to select for cross-reactive antibodies. In particular, HCV-I002 blood samples were obtained from confirmed HCV-infected patients, and peripheral blood mononuclear cells (Peripheral bloodmononuclear cells, PBMCs) were separated by lymphocyte separation tubes using a BD FACSria flow cytometer (BD Biosciences, San Jose, CA ) for cell sorting to obtain antigens specifically labeled with fluorescein to obtain E2 double fluorescently labeled target cells. Briefly, with this approach it is possible to obtain antibodies that are produced during natural infection but are still able to bind different glycoproteins that have never been encountered by the immune system o...
Embodiment 2
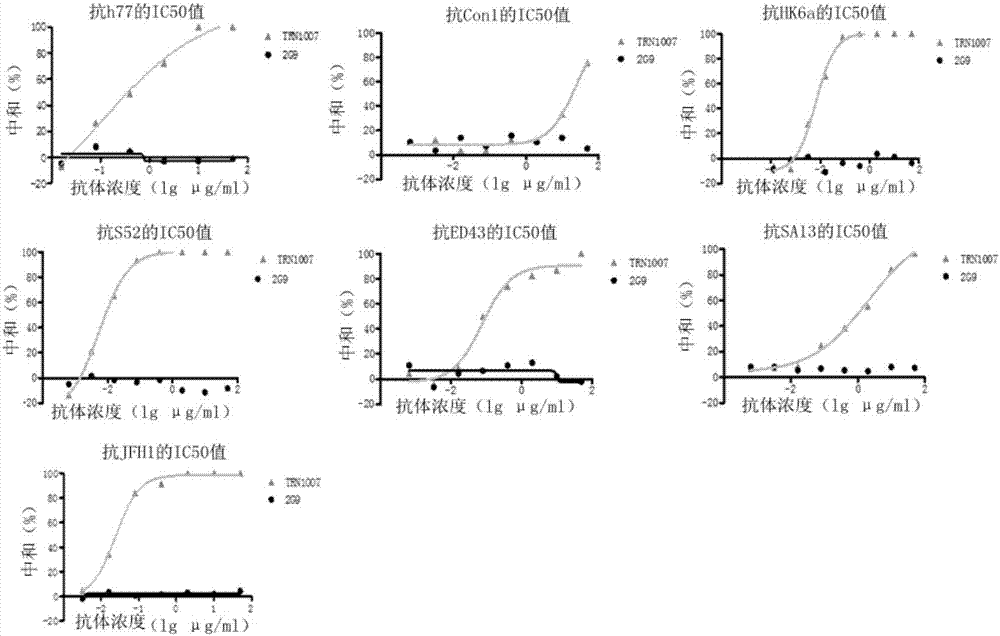
[0053] Example 2 Evaluation of neutralizing activity of TRN1007 antibody and different HCV true virus strains
[0054] Briefly, Huh7 cells were plated in 96-well plates and cultured overnight, and the cells were approximately 30% confluent at the time of infection. HCV true virus strains (h77, Con1, JFH1, S52, ED43, SA13, HK6a) were mixed with different concentrations of antibodies, the initial concentration was 25 μg / ml, diluted 3 times, and left at room temperature for 30 minutes. Add HCV true virus strains (h77, Con1, JFH1, S52, ED43, SA13, HK6a) and antibody mixture to a 96-well plate to infect Huh7 cells. In the control group, only HCV virus strain 2G9 was added, the medium was changed after 24 hours of infection, and the culture was continued for 1-2 days. The activity was measured 2-3 days after infection, and the fluorescence value was read. The neutralization efficiency was calculated by comparing the antibody with the control group.
[0055] The results are shown ...
Embodiment 3
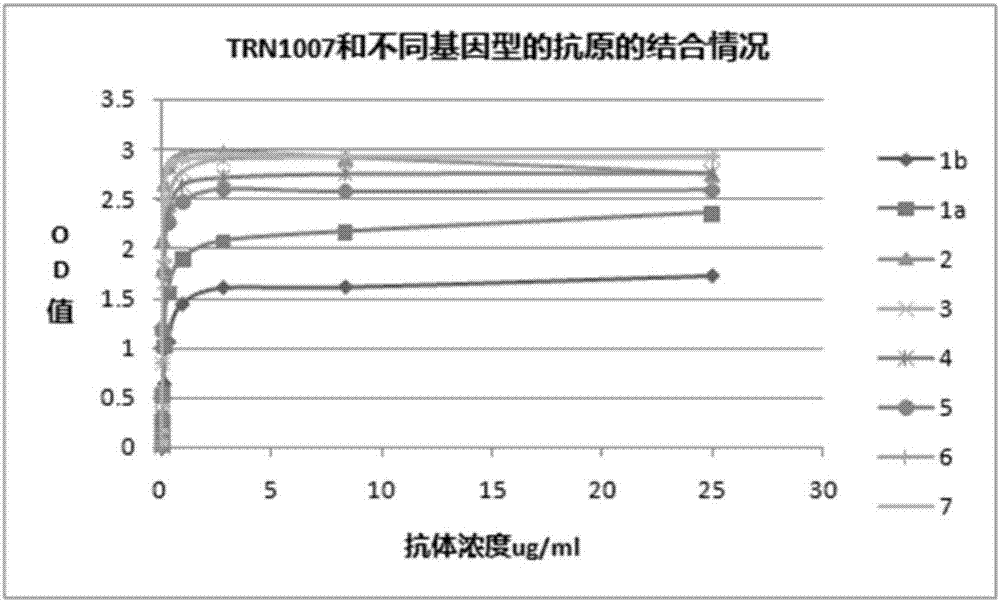
[0058] Example 3 Assessment of Binding Activity of TRN1007 Antibody to HCV Antigens from Different Genotypes
[0059] Use different HCV strains (1a, 1b, 2, 3, 4, 5, 6, 7) membrane protein E2 as antigen, and use coating solution to dilute the antigen to a concentration of 100ng / ml, and coat it on an ELISA 96-well plate, 100 μl per well, overnight at 4°C. The blocking solution was blocked for 2 hours at 37°C. After blocking, add the primary antibody, the initial concentration is 25 μg / ml, 3-fold serial dilution, the volume of each well is 100 μl, and incubate at 37°C for 1 hour. At the same time, HCV positive patient serum is used as a positive control, and rabies antibody is used as a negative control. HRP-labeled goat anti-human IgG (diluted 1:2000) was used as a secondary antibody and incubated at 37°C for 1 hour. Add 100 μL / well of substrate chromogenic solution (TMB), place at 37°C in the dark for 5 min, stop the reaction with 2M sulfuric acid, and perform colorimetry wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com