Lithium battery electrolyte based on sulfate additive
An additive and sulfate technology, applied in the field of lithium battery electrolyte and new energy materials, can solve the problems of dendrite growth and low coulombic efficiency, and achieve the effect of easy large-scale continuous production, easy manufacturing and strong universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: 1M LiPF with added dimethyl sulfate 6 Lithium deposition / deposition efficiency of EC / DMC (v=1:1) electrolyte
[0039] In an inert atmosphere, add 1 wt% dimethyl sulfate to commercially available 1M LiPF 6 EC / DMC (v=1:1) solution to prepare the electrolyte solution for use. According to the order of the positive battery shell, copper pole piece, separator, lithium metal pole piece, and negative battery shell, inject the electrolyte to be used, and assemble it into a button battery in the glove box.
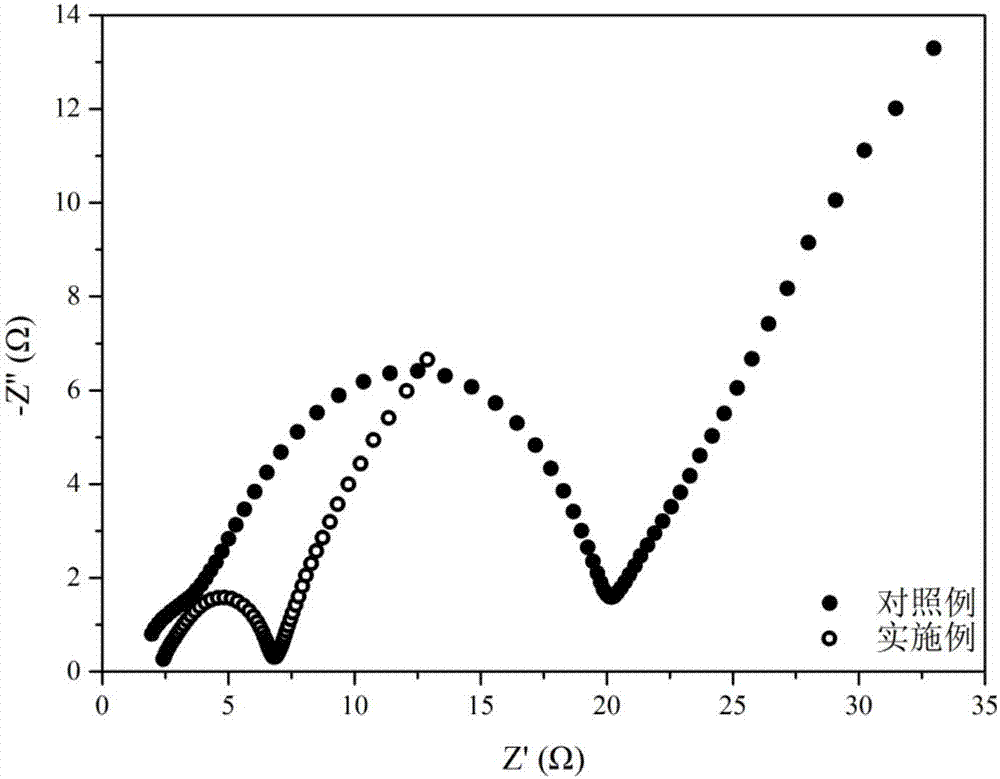
[0040] The assembled button battery constitutes a copper and lithium two-electrode system. The lithium metal pole piece is used as a reference and auxiliary electrode, and the copper pole piece is used as a working electrode. The deposition and precipitation process of lithium metal on the current collector can be investigated. By analyzing this process , the reversibility and efficiency of the deposition and precipitation process can be judged, so as to exami...
Embodiment 2
[0042] Example 2: 1M LiPF with added dimethyl sulfate 6 Scanning electron microscope topography of lithium deposition / precipitation in EC / DMC (v=1:1) electrolyte
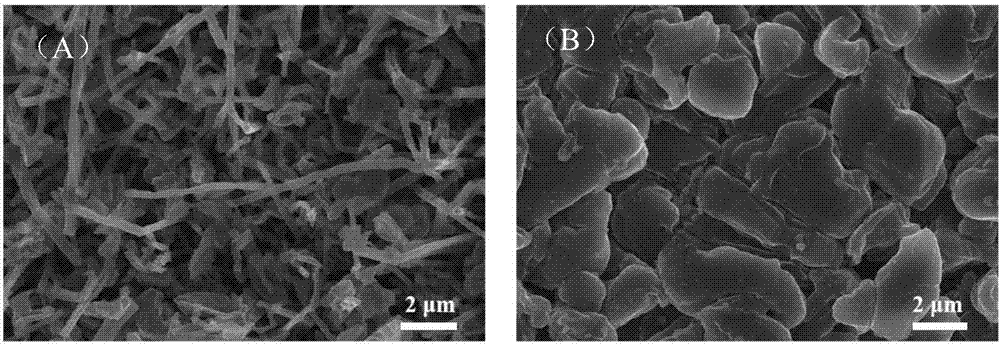
[0043] Using the method of Example 1, the button cell after 20 weeks of circulation was disassembled in the glove box, the copper pole pieces were taken out, washed with dimethyl carbonate, dried, and placed under a scanning electron microscope for observation, the results Such as image 3 shown. The control group without adding sulfate additives showed a typical lithium deposition morphology. Acicular needle-like dendrites with a diameter of about 200nm were deposited on the copper current collector. The growth of dendrites in this form would pierce the separator and cause battery internal damage. short circuit, causing serious safety problems. After adding the sulfate additive, after the electrolyte was circulated for 20 weeks, the morphology of the lithium deposition was dendrites about 5 μm nodular particles...
Embodiment 3
[0044] Example 3: 1M LiTFSI DOL / DME (v=1:1)+1% LiNO with added diethyl sulfate 3 Lithium deposition / deposition efficiency of electrolyte
[0045] In an inert atmosphere, add diethyl sulfate to 1M LiTFSI DOL / DME (v=1:1)+1% LiNO 3 In the electrolyte, its mass fraction is 3.8%. Li|Cu button cells were assembled according to the method in Example 1, and the electrochemical performance test was carried out.
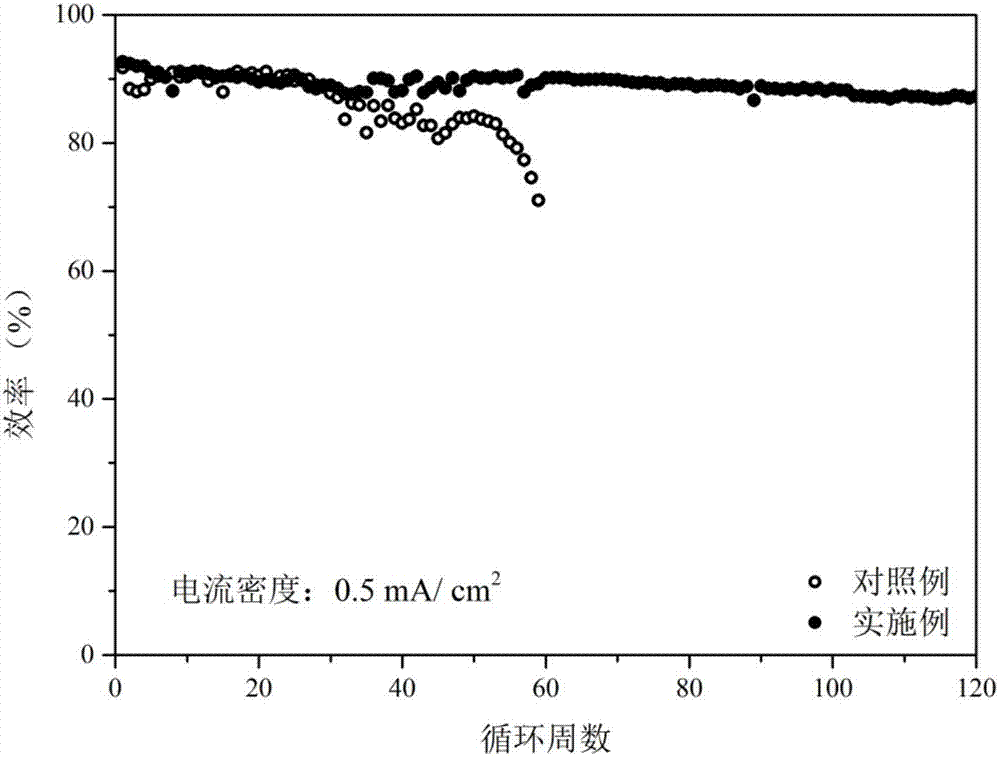
[0046] Figure 4 It is 1M LiTFSI DOL / DME (v=1:1)+1% LiNO with added diethyl sulfate 3 Electrolyte Li|Cu battery cycle life diagram. From Figure 4 It can be seen that the deposition / precipitation efficiency of the blank control example is only 91.2% in the first week, while the electrolyte solution added with diethyl sulfate additive improves the efficiency to 95.1% in the first week. At the same time, the number of Li|Cu battery cycles increased from 40 to 100 cycles with the addition of diethyl sulfate electrolyte, an increase of 2.5 times. Prove that the sulfate este...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com