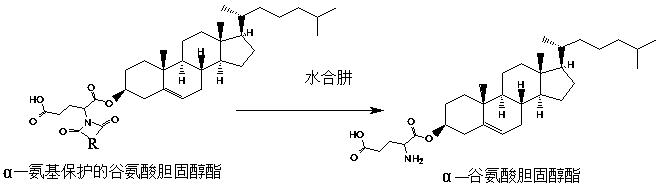
A kind of preparation method of α-cholesteryl glutamate gel
A technology of cholesteryl ester and glutamic acid, which is applied in the directions of gel preparation, organic chemistry method, chemical instrument and method, etc., can solve the problems of long reaction time, difficult reaction and low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] Concrete preparation method steps are as follows:
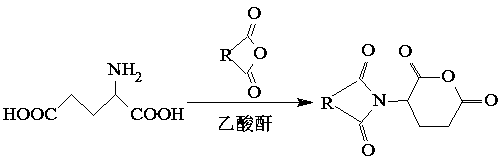
[0021] Step 1. Weigh 2-5g of α-glutamic acid and mix it with acid anhydride with a mass ratio of 1:1, heat and melt, then add 5-10ml of acetic anhydride, reflux at 120-140°C for 10-30min, and let stand after the reaction , cooled to room temperature, precipitated crystals, filtered, washed the crystals three times with ethyl acetate, and dried in vacuum at 60°C to obtain α-amino-protected glutamic anhydride.
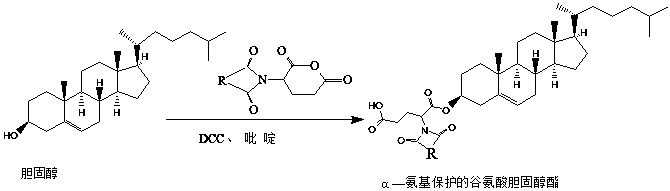
[0022] Step 2, add cholesterol to the product prepared above according to its molar ratio of 1:1-3:1, add 60ml of dichloromethane to fully dissolve and dissolve, add catalyst 0.5-1.2g dicyclohexylcarbodiimide (DCC), 2-4ml of pyridine was stirred at room temperature for 4 hours, filtered, and the filter residue was discarded. The filtrate was washed with 0.01mol / L hydrochloric acid, 0.01mol / L sodium hydroxide and deionized water to neutrality, and rotary evaporated, and the obtained solid was washed with hot metha...
Embodiment 1
[0036] (1) Weigh 2g of α-glutamic acid and mix it with succinic anhydride with a mass ratio of 1:1, heat and melt, then add 5ml of acetic anhydride, reflux at 120°C for 30min, let stand after the reaction, cool to room temperature, and precipitate The crystals were filtered, washed three times with ethyl acetate, and dried under vacuum at 60°C to obtain α-amino-protected glutamic anhydride.
[0037] (2) Add cholesterol to the product prepared above at a molar ratio of 2:1, add 60ml of dichloromethane to fully dissolve, add catalyst 0.5g DCC, 3ml of pyridine, stir at room temperature for 4h, filter, and discard the filter residue. The filtrate was washed with 0.01mol / L of hydrochloric acid, 0.01mol / L of sodium hydroxide and deionized water to neutrality, and then evaporated by rotary evaporation. The resulting solid was washed three times with hot methanol, and the crude product was separated and purified by silica gel chromatography (ethyl acetate Esters: cyclohexane = 1:4) to...
Embodiment 2
[0040](1) Weigh 5g of α-glutamic acid and mix it with phthalic anhydride with a mass ratio of 1:1, heat and melt, then add 10ml of acetic anhydride, reflux at 140°C for 20min, let stand after the reaction, and cool to room temperature , precipitated crystals, filtered, washed the crystals with ethyl acetate three times, and dried in vacuum at 60°C to obtain α-amino-protected glutamic anhydride.
[0041] (2) Add cholesterol to the product prepared above at a molar ratio of 3:1, add 60ml of dichloromethane to fully dissolve, add catalyst 1.2g of DCC, 4ml of pyridine, stir at room temperature for 4h, filter, and discard the filter residue. The filtrate was washed with 0.01mol / L of hydrochloric acid, 0.01mol / L of sodium hydroxide and deionized water to neutrality, and then evaporated by rotary evaporation. The resulting solid was washed three times with hot methanol, and the crude product was separated and purified by silica gel chromatography (ethyl acetate Esters: cyclohexane = ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com