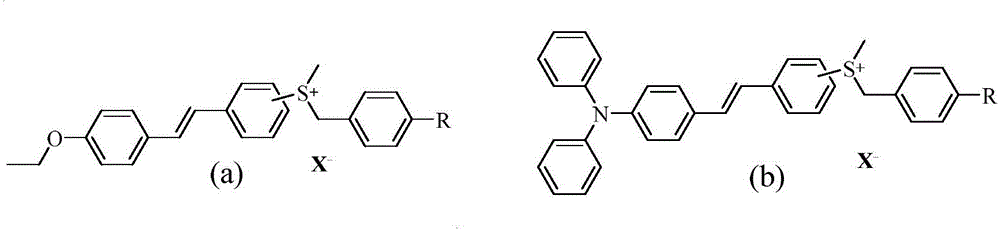
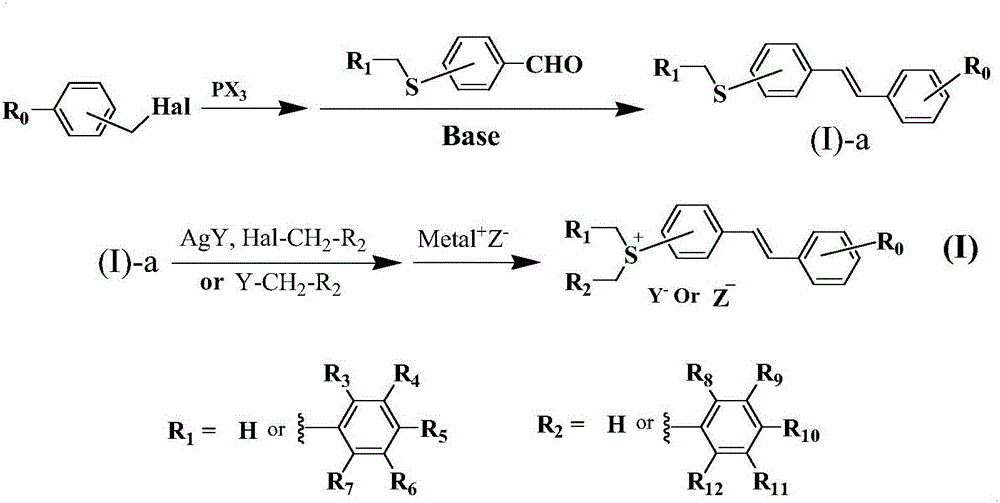
Novel preparation method of sulfonium salt derivatives with stilbene as conjugated system
A technology of stilbene and conjugated systems, which is applied in the field of preparation of sulfonium salt derivatives, can solve the problems of expensive raw materials and long reaction steps, and achieve the effects of convenient purification, simple operation and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
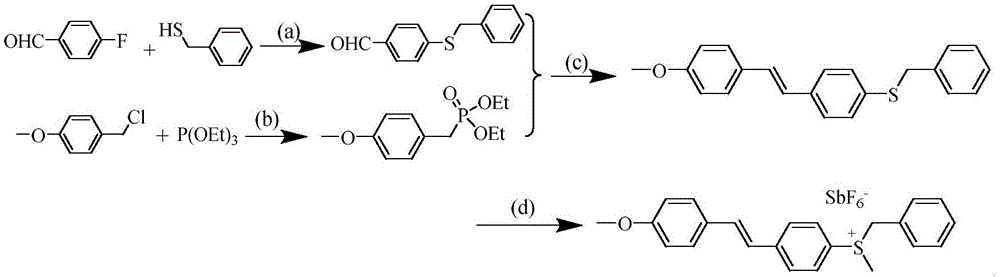
[0023] Example 1: Synthesize the target sulfonium salt molecule containing methoxy group according to the following route
[0024]
[0025] (a): K 2 CO 3 , DMF, 100°C, 4h;
[0026] (b): reflux, 4h;
[0027] (c): NaH, DMF, room temperature, 12h;
[0028] (d): Methyl trifluoromethanesulfonate (or dimethyl sulfate); sodium hexafluoroantimonate, dichloromethane, room temperature, 24h.
[0029] 1. Synthesis of 4-benzylmercaptobenzaldehyde
[0030] In a 250mL three-neck flask, add K 2 CO 3 (20.37g, 0.15mol) and 75mL DMF, vacuum the system and fill with N 2 After three times, it was placed in an oil bath at 100°C, and 4-fluorobenzaldehyde (12.20 g, 0.1 mol) and benzyl mercaptan (12.40 g, 0.1 mol) were injected sequentially, respectively, and stirred for 4 h. Slowly add the system to 10 times the volume of distilled water with stirring, collect the precipitate by filtration, and store it in a vacuum oven at 40°C. Yield 91.0%.
[0031] 1 H NMR (400MHz, CDCl 3 )δ: 9.91(s,...
Embodiment 2
[0038] Example 2: The target sulfonium salt molecule containing N,N-diphenylamino was synthesized according to the following route
[0039]
[0040] (a): toluene, reflux, 24h;
[0041] (c): Potassium tert-butoxide, THF, room temperature, 12h;
[0042] (d): Methyl trifluoromethanesulfonate, dichloromethane, room temperature, 24h.
[0043] 1. Synthesis of Phosphate Salt
[0044] Take a dry 250mL round bottom flask, add 4-N,N-dimethylaminobenzyl bromide 3.38g (10mmol, 1equ) to it, and 30mL anhydrous toluene, then add 2.02g (10mmol, 1equ) to the reaction system 1equ) Tri-n-butylphosphine. The reaction was refluxed for 24h. After the reaction was over, the reaction flask was lifted and cooled to room temperature. Then put the flask into the refrigerator to freeze for 2h. After freezing, a white solid appeared in the flask, and it was suction-filtered at low temperature and washed twice with toluene to obtain 4.84 g of a white powder product with a yield of 70%.
[0045] ...
Embodiment 3
[0055] Embodiment 3: Synthesize the target sulfonium salt molecule containing a methyl group according to the following route
[0056]
[0057] (a): K 2 CO 3 , DMF, 100°C, 4h;
[0058] (b): reflux, 4h;
[0059] (c): NaH, DMF, room temperature, 12h;
[0060] (d): Methyl trifluoromethanesulfonate (or dimethyl sulfate), potassium hexafluorophosphate, dichloromethane, room temperature, 24h.
[0061] 1. Synthesis of 4-benzylmercaptobenzaldehyde
[0062] The steps are the same as the synthesis process in Example 1.
[0063] 1 H NMR (400MHz, CDCl 3 )δ: 9.91 (s, 1H), 7.74 (d, 2H), 7.41-7.27 (m, 7H), 4.24 (s, 2H).
[0064] 2. Synthesis of 4-(4-methylphenyl)vinylphenylbenzyl sulfide
[0065] Take the reaction product (2.91g, 12mmol) of p-methylbenzyl chloride and excess triethyl phosphite in a three-necked flask, add NaH (1.23g, 50mmol) and 100mL DMF at room temperature, find that there is gas generation, and then add 4 -Benzylmercaptobenzaldehyde (2.25g, 10mmol), after sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com