A kind of preparation method of magnesium sulfate heptahydrate
A technology of magnesium sulfate heptahydrate and bischofite, which is applied in the direction of magnesium sulfate, can solve the problems of high impurity ion content and high reaction energy consumption, and achieve the effect of simple process flow, simple reaction process and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
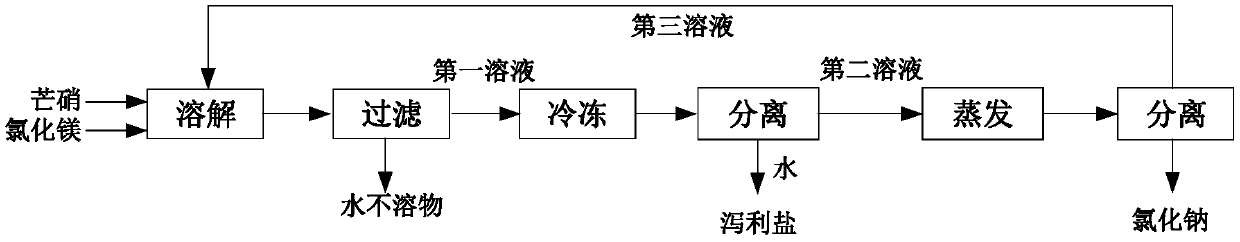
[0026] A, Glauber's salt and bischofite are proportioned according to the mass ratio of 1:3.6, add water to dissolve, control the water content of the system to be 72.2%, and obtain the first solution;
[0027] B, the insoluble matter of the first solution is removed by filtration;
[0028] C. Freeze the filtered first solution, control the freezing temperature to 0° C., and freeze for 72 hours to crystallize magnesium sulfate heptahydrate to obtain the second solution.
[0029] D. Evaporate the second solution to obtain sodium chloride, and the evaporated water is 40%.
[0030] Sulfate root freezing out rate is 28.6%, heavy metal ion is not detected in magnesium sulfate heptahydrate, potassium ion content is 0.02%, boron ion content is 0.008%, chloride ion content is 0.5%, sodium ion content is 0.21%, heptahydrate The magnesium sulfate content is 99.20%. The frozen mother liquor evaporates 40% of the water, and the sodium chloride precipitation rate is 92%, wherein the cont...
Embodiment 2
[0032] A, Glauber's salt and bischofite are proportioned according to the mass ratio of 1:7.8, add water to dissolve, control the water content of the system to be 66%, and obtain the first solution;
[0033] B, the insoluble matter of the first solution is removed by filtration;
[0034] C. Freeze the filtered first solution, control the freezing temperature to -20° C., and freeze for 12 hours to crystallize magnesium sulfate heptahydrate to obtain the second solution.
[0035] D. Evaporate the second solution to obtain sodium chloride, and the evaporated water is 30%.
[0036] The freezing-out rate of sulfate radicals was 46.2%, no heavy metal ions were detected in the magnesium sulfate heptahydrate, and the content of chloride ions was 1%. When the washing amount is 13% of the quality of magnesium sulfate heptahydrate, the content of potassium ion in magnesium sulfate heptahydrate is 0.007%, the content of boron ion is 0.008%, the content of chloride ion is 0.15%, the cont...
Embodiment 3
[0038] A, Glauber's salt and bischofite are proportioned according to the mass ratio of 1:5, add water to dissolve, control the water content of the system to be 74%, and obtain the first solution;
[0039] B, the insoluble matter of the first solution is removed by filtration;
[0040] C. Freeze the filtered first solution, control the freezing temperature to -10°C, and freeze for 24 hours to crystallize and precipitate magnesium sulfate heptahydrate to obtain the second solution.
[0041] D. Evaporate the second solution to obtain sodium chloride, and the amount of evaporated water is 20%.
[0042] The mass ratio of Glauber's salt to bischofite was selected as 1:5, the freezing temperature was -10°C, the water content of the system was controlled at 74%, and after freezing for 24 hours, the sulfate radical freezing rate was 50.6%, which was not detected in magnesium sulfate heptahydrate Heavy metal ions, chloride ion content is 0.45%. When the washing amount is 25% of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com