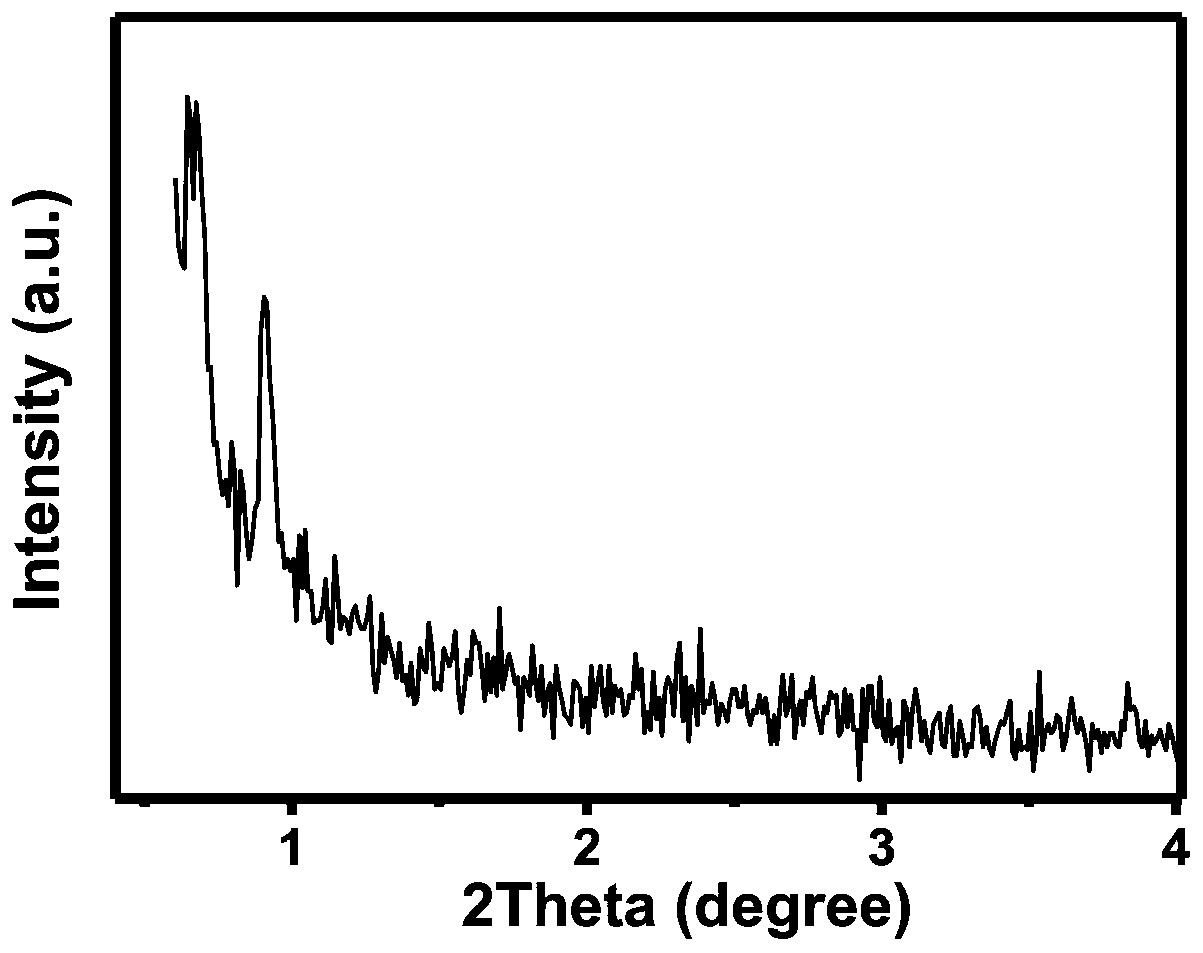
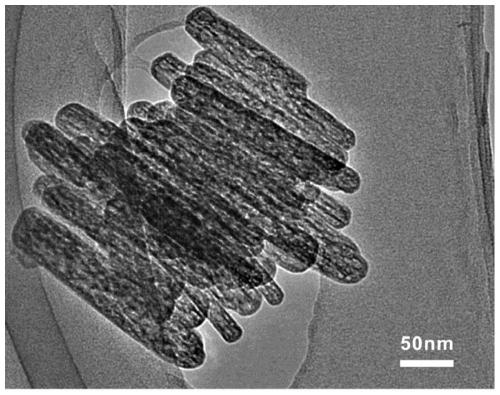
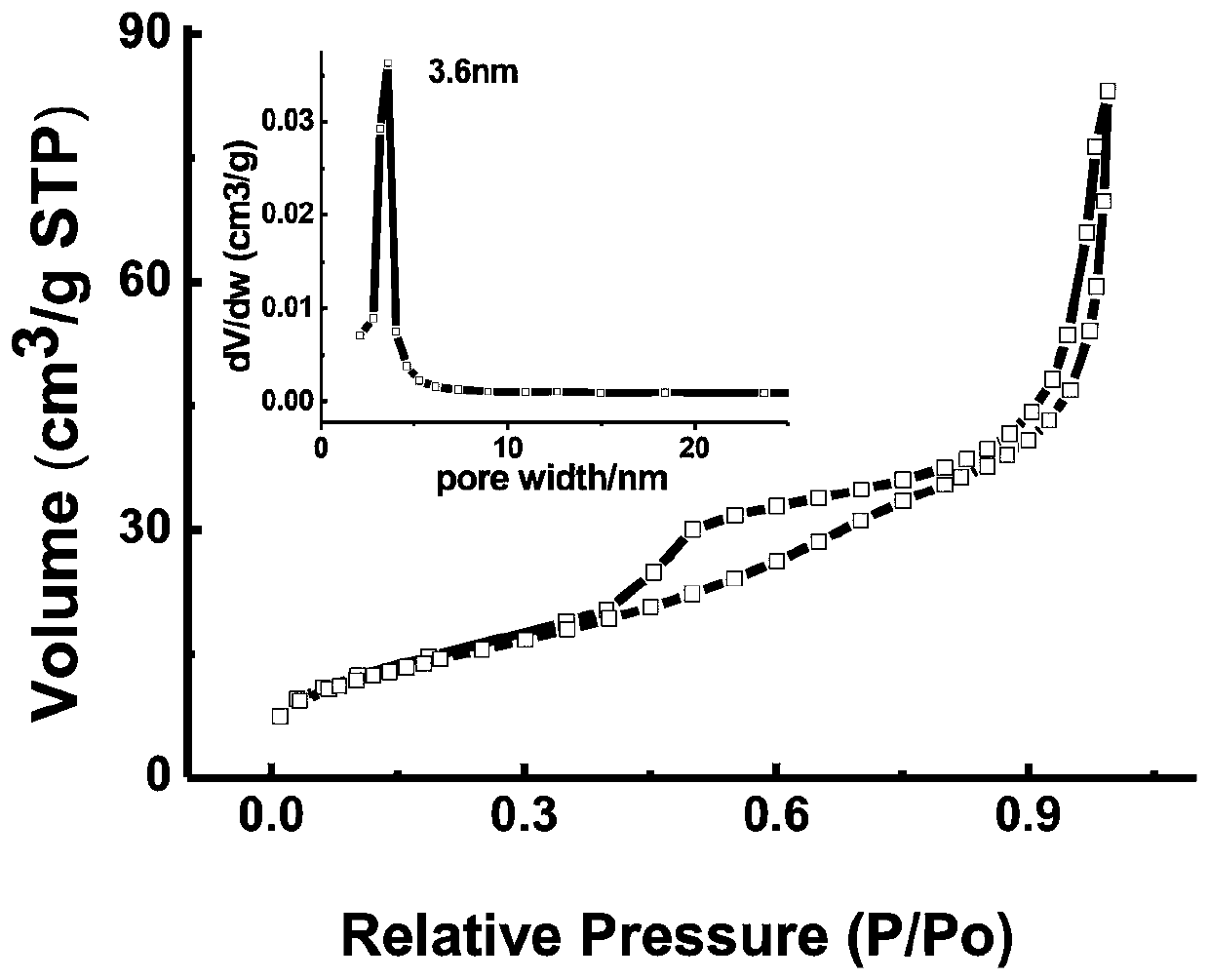
A preparation method of ordered mesoporous iron oxyhydroxide nanorods
An iron oxide nanometer, mesoporous hydroxyl technology, applied in the direction of iron oxide/iron hydroxide, etc., can solve the problems of complex crystal phase, skeleton collapse, affecting the order degree of mesopores, etc. Inexpensive and readily available effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Dissolve 0.5g of non-ionic block copolymer surfactant in 35mL of distilled water, add anhydrous ferric chloride, stir and disperse fully to obtain a yellow solution.
[0036] (2) While stirring the yellow solution, 0.5 g of urea precipitation agent was added dropwise at a rate of 1.5 mL / min, and stirred at a constant temperature of 30 ° C for 12 h to obtain a precursor solution.
[0037] (3) Transfer the precursor solution to a reaction kettle with a polytetrafluoroethylene liner, conduct a hydrothermal reaction at 80°C, and centrifuge to collect the precipitate. After the precipitate is fully washed with ethanol and water, it is vacuum-dried overnight at 100°C. , to obtain solid powder.
[0038] (4) Transfer the solid powder to a crucible, and in an inert atmosphere, start from room temperature at a rate of 5 °C / min to 150 °C, keep the temperature for 2.5 h, and cool down naturally to obtain ordered mesoporous iron oxyhydroxide nanorods.
Embodiment 2
[0040](1) Dissolve 2 g of the nonionic block copolymer surfactant PEOPPOPEO in 80 mL of distilled water, add ferric nitrate, stir and disperse fully to obtain a yellow solution.
[0041] (2) While stirring the yellow solution, 2 g of urea precipitation agent was added dropwise at a rate of 3 mL / min, and stirred at a constant temperature of 60 °C for 48 h to obtain a precursor solution.
[0042] (3) Transfer the precursor solution to a reaction kettle with a polytetrafluoroethylene liner, react hydrothermally at 120°C, centrifuge to collect the precipitate, wash the precipitate fully with ethanol and / or water, and vacuum at 100°C Dry overnight to obtain a solid powder.
[0043] (4) Transfer the solid powder into a crucible, and in an inert atmosphere, start from room temperature at a rate of 5 °C / min to 250 °C, keep it warm for 2.5 h, and cool down naturally to obtain ordered mesoporous iron oxyhydroxide nanorods.
Embodiment 3
[0045] (1) Dissolve 1 g of non-ionic block copolymer surfactant in 45 mL of distilled water, add hydrous ferric chloride, stir and disperse fully to obtain a yellow solution.
[0046] (2) While stirring the yellow solution, 1.5 g of urea precipitation agent was added dropwise at a rate of 2 mL / min, and stirred at a constant temperature of 40 °C for 24 h to obtain a precursor solution.
[0047] (3) Transfer the precursor solution to a reaction kettle with a polytetrafluoroethylene liner, conduct a hydrothermal reaction at 120°C, centrifuge to collect the precipitate, wash the precipitate with ethanol or water, and dry it under vacuum at 100°C overnight , to obtain solid powder.
[0048] (4) Transfer the solid powder to a crucible, and in an inert atmosphere, start from room temperature at a rate of 5 °C / min to 200 °C, keep it warm for 2.5 h, and cool down naturally to obtain ordered mesoporous iron oxyhydroxide nanorods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com