A Simple System Electrodeposition Zinc Coating Method
An electrodeposition and system technology, applied in the field of simple system electrodeposition zinc coating, can solve problems such as pollution of the environment, and achieve the effect of simple raw materials and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Weigh 27.92g of choline chloride and 40.89g of zinc chloride at a molar ratio of 1:1.5, put the two into a flask and mix them with a glass rod, then fix the flask in an oil bath and place Stir and heat on a magnetic stirrer, control the temperature at 90°C until a transparent viscous liquid is formed, and finally obtain 50ml of choline chloride-zinc chloride ion solution;
[0023] (2) Choose zinc sheet as soluble anode, copper block as cathode, put into the ionic solution prepared in (1) above to carry out constant current electrodeposition reaction, the distance between cathode and anode is 2cm, control temperature is 120 ℃, reaction time 40min, the current density is 2 mA / cm 2 ;
[0024] (3) After electrodeposition, the samples were taken out and ultrasonically cleaned with anhydrous ethanol and deionized water respectively, and finally dried with a hair dryer at low temperature, and the temperature was controlled at 25°C.
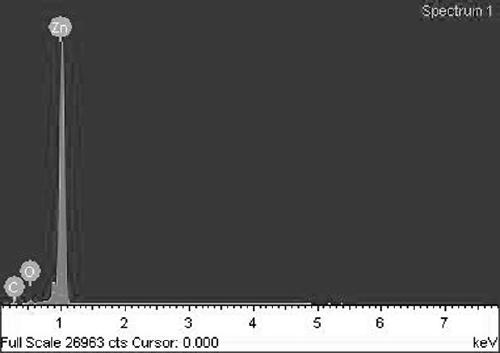
[0025] The surface energy spectrum an...
Embodiment 2
[0027] (1) Weigh 27.92g of choline chloride and 54.52g of zinc chloride at a molar ratio of 1:2, put the two into a flask and mix well with a glass rod, then fix the flask in an oil bath and place Stir and heat on a magnetic stirrer, control the temperature at 90°C until a transparent viscous liquid is formed, and finally obtain 60ml of choline chloride-zinc chloride ion solution;
[0028] (2) Select zinc sheet as soluble anode, copper block as cathode, put into the ionic solution prepared in (1) above to carry out constant current electrodeposition reaction, the distance between cathode and anode is 2cm, control temperature is 110 ℃, reaction time 30min, the current density is 4 mA / cm 2 ;
[0029] (3) After electrodeposition, the samples were taken out and ultrasonically cleaned with anhydrous ethanol and deionized water respectively, and finally dried with a hair dryer at low temperature, and the temperature was controlled at 25°C.
[0030] The SEM image of the surface of ...
Embodiment 3
[0032] (1) Weigh 27.92g of choline chloride and 68.15g of zinc chloride at a molar ratio of 1:2.5, put the two into a flask and mix them with a glass rod, then fix the flask in an oil bath and place Stir and heat on a magnetic stirrer, control the temperature at 90°C until a transparent viscous liquid is formed, and finally obtain 70ml of choline chloride-zinc chloride ion solution;
[0033] (2) Choose zinc sheet as soluble anode, copper block as cathode, put into the ionic solution prepared in (1) above to carry out constant current electrodeposition reaction, the distance between cathode and anode is 2cm, control temperature is 140 ℃, reaction time 20min, the current density is 6 mA / cm 2 ;
[0034] (3) After electrodeposition, the samples were taken out and ultrasonically cleaned with anhydrous ethanol and deionized water respectively, and finally dried with a hair dryer at low temperature, and the temperature was controlled at 25°C.
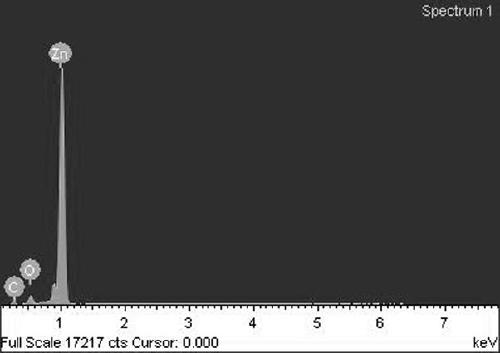
[0035] The surface energy spectrum an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com