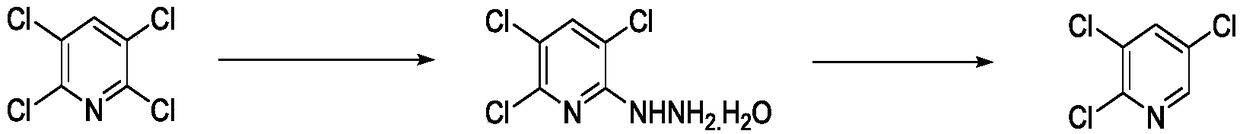
Method for synthesizing 2, 3, 5-trichloropyridine
A technology of trichloropyridine and a synthesis method, applied in the field of pesticide chemical industry, can solve the problems of complicated synthesis process operation, unsatisfactory product yield, harsh reaction conditions, etc., and achieves the effects of high product yield, less three wastes and thorough reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0027] Example 1
[0028] Add 60g of methanol into a 1000mL four-necked flask, put in 100g of 2,3,5,6-tetrachloropyridine, 31.7g of hydrazine hydrate, raise the temperature to 60~65℃, keep the temperature for about 2 hours, the reaction is over, and the temperature is reduced to 0~5 Stir at ℃ for 1 hour, filter, and the solid is 2,3,5-trichlorohydrazinopyridine 6-hydrate, dried to obtain 101.6g of white solid, yield 96%, content 98.5%.
[0029] Add 100g of 2,3,5-trichlorohydrazinopyridine 6-hydrate to a 1000ml four-necked flask, 50g of 5% sodium hydroxide aqueous solution, raise the temperature to 70~75℃, drop 387.6g of 10% sodium hypochlorite aqueous solution, Incubate at 70~75℃, react for 1 hour, the reaction is over, cool to 5~10℃, stir for 1 hour, filter to obtain crude 2,3,5-trichloropyridine, and distill under reduced pressure to obtain the product as a light yellow solid. Rate: 95%, content: 98%.
Example Embodiment
[0030] Example 2
[0031] Add 50g of methanol into a 1000mL four-necked flask, put in 100g of 2,3,5,6-tetrachloropyridine, 34.6g of hydrazine hydrate, raise the temperature to 55~60℃, keep the temperature and react for about 2 hours, the reaction is over, and the temperature is reduced to 0~5 Stir for 1.5 hours at ℃, filter, the solid is 2,3,5-trichlorohydrazinopyridine 6-hydrate, and dry to obtain 101.5g of white solid with 95.8% yield and 98.5% content.
[0032] Add 100g of 2,3,5-trichlorohydrazinopyridine 6-hydrate to a 1000ml four-neck bottle, 45g of 5% sodium hydroxide aqueous solution, heat to 70~75℃, drop 355.3g of 10% sodium hypochlorite aqueous solution, Incubate at 70~75℃, react for 1 hour, the reaction is over, cool to 5~10℃, stir for 1 hour, filter to obtain crude 2,3,5-trichloropyridine, and distill under reduced pressure to obtain the product as a light yellow solid. Rate: 93%, content: 98%.
Example Embodiment
[0033] Example 3
[0034] Add 60g of methanol into a 1000mL four-necked flask, put in 100g of 2,3,5,6-tetrachloropyridine, 33.1g of hydrazine hydrate, raise the temperature to 60~65℃, keep the temperature for about 2 hours, the reaction is over, and the temperature is reduced to 0~5 Stir for 1 hour at ℃, filter, and the solid is 2,3,5-trichlorohydrazinopyridine 6-hydrate, and dried to obtain 101.6g of white solid with a yield of 96% and a content of 98.4%.
[0035] Add 100g of 2,3,5-trichlorohydrazinopyridine 6-hydrazine, 57g of 5% sodium hydroxide aqueous solution to a 1000ml four-necked flask, heat up to 70~75℃, dropwise add 360g of 10% sodium hypochlorite aqueous solution, keep warm 70~75℃, react for 1 hour, the reaction is over, cool to 5~10℃, stir for 1 hour, filter to obtain crude 2,3,5-trichloropyridine, and distill under reduced pressure to obtain the product as a pale yellow solid. Yield : 94.5%, content: 98%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap