Application of avobenzone in preparing antitumor drugs
An anti-tumor drug, the technology of avobenzone, applied in the field of medicine, can solve the problems of researching and verifying the significance of skin cancer treatment, without the perspective of cell biology, and without verifying the therapeutic effect of avobenzone on skin cancer, so as to reduce the Clinical drug resistance, side effect response measures are clear, and the effect of improving application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
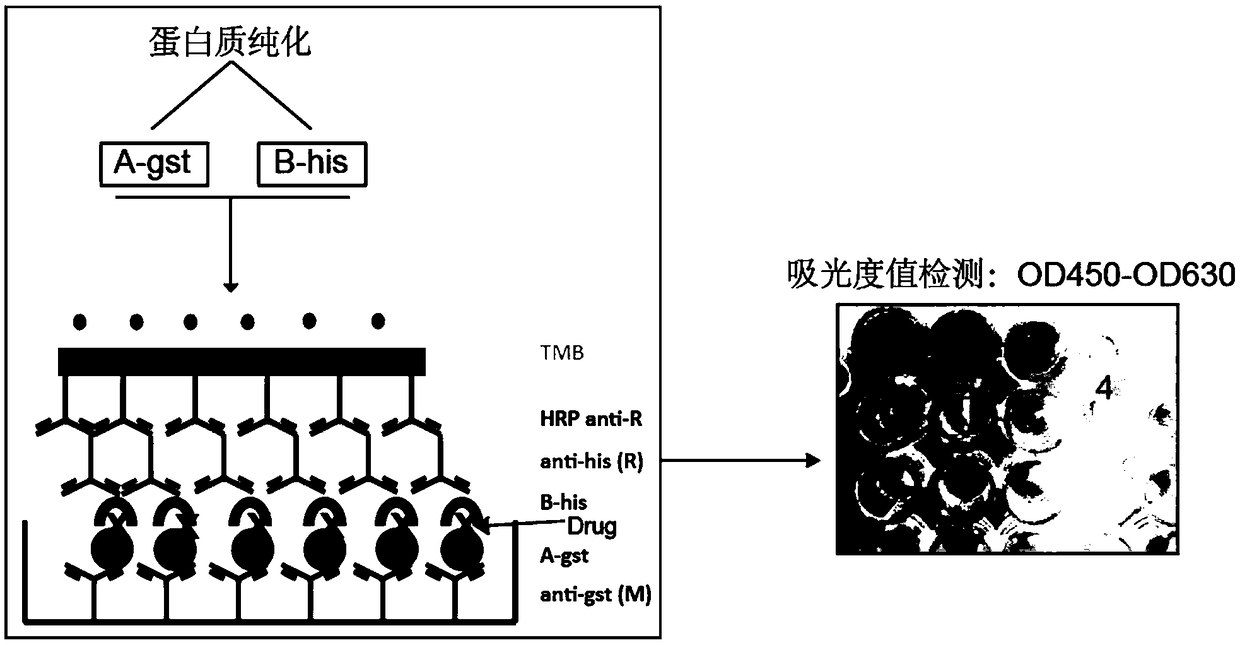
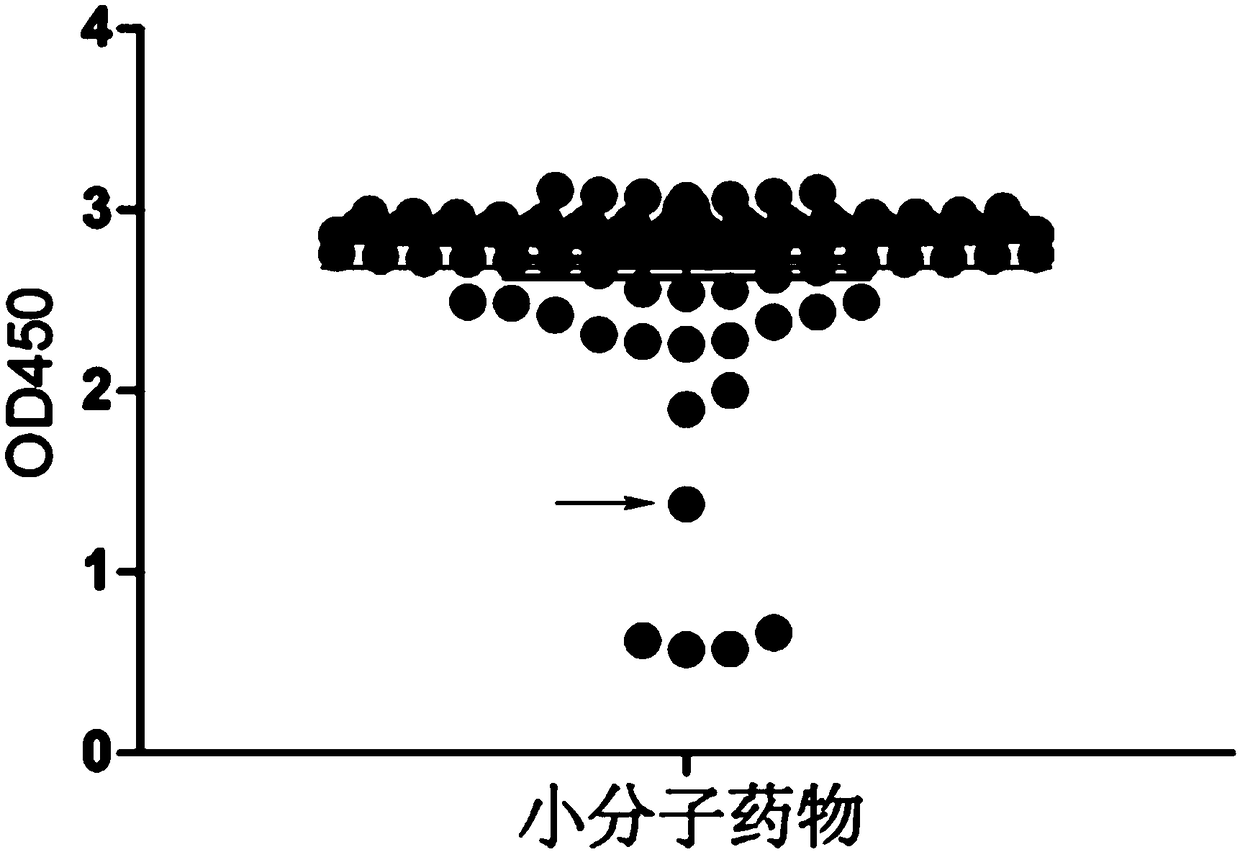
[0026] Example 1 Research on the Anti-tumor Mechanism of Avobenzone by SuperELISA Technology
[0027] 1. Preparation of fusion protein PHB1-his, C-Raf1-gst
[0028] (1) Expression and purification of PHB1-his fusion protein
[0029] 1. Construction of the recombinant expression vector pET-his: according to the human PHB1 protein gene sequence in the NCBI database (NCBI accession number: NM_001281496.1), use Primer Premier 5.0 to design and synthesize primers encoding the PHB1 gene, and add double restriction sites at both ends (The restriction site at the upstream end is BamH1, and the restriction site at the downstream end is EcoR1, both purchased from TAKARA Company), and the target gene band is amplified by PCR.
[0030] 2. The PCR product was detected by 1% agarose gel electrophoresis, and the target fragment was recovered and purified using a DNA recovery kit (Tiangen Biochemical Technology Co., Ltd., catalog number: DP214). Use endonucleases to double-digest the target...
Embodiment 2
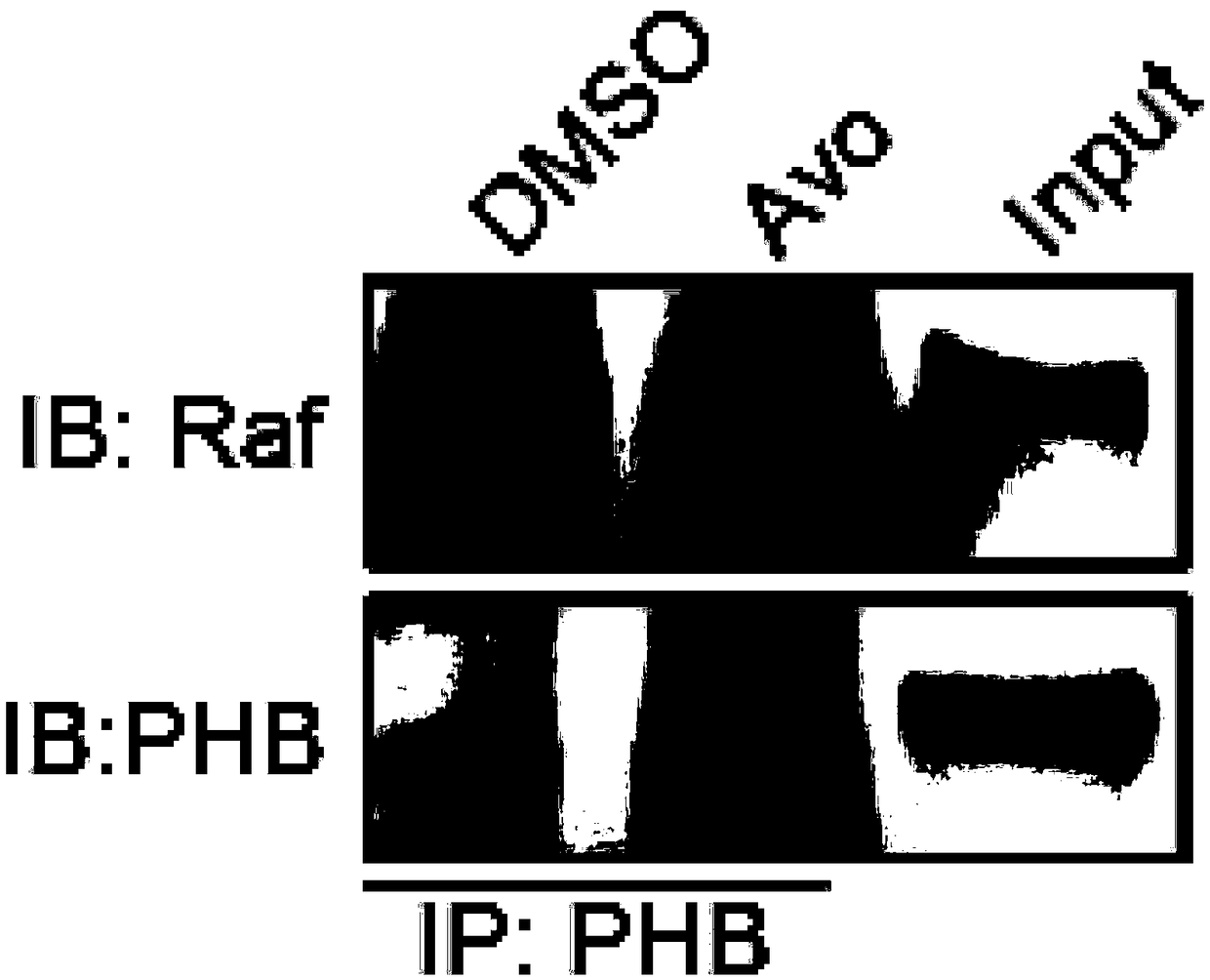
[0070] Example 2 Co-immunoprecipitation experiment
[0071]Co-immunoprecipitation experiment (Co-IP) is an important means to test the interaction of proteins in vivo. In order to further study and verify the anti-cancer mechanism and effect of avobenzone, this embodiment carried out co-immunoprecipitation experiment (Co-IP) on avobenzone The role of Zong should be further studied. The specific operation steps of Co-IP are as follows:
[0072] (1) Spread 2 groups of 1 million colon cancer HCT116 cells in a cell culture dish in advance, and add DMSO and avobenzone respectively. DMSO was added according to the ratio of 1 μL: 1000 μL DMEM culture solution, avobenzone was diluted with DMEM culture solution, and the final concentration was 10 μM;
[0073] (2) Cultivate the treated cells at 37°C for 24 hours, then wash the cells twice with PBS, add 1 mL of 0.25% trypsin to each culture dish to digest the cells to make them fall off the wall of the culture dish; then terminate with...
Embodiment 3
[0082] Example 3 MEK activation level research
[0083] The protein MEK is a downstream molecule of the PHB-c-Raf1 signaling pathway, and whether the interaction between PHB and c-Raf1 is destroyed can be confirmed by detecting the activation level of MEK, that is, the phosphorylation state (pMEK). Reduced levels of pMEK also imply reduced metastatic ability of colon cancer cells.
[0084] The activation level of MEK was detected by western blot method, and the specific steps were as follows:
[0085] 1. Spread colon cancer cells HCT116 or colon cancer cells RKO (purchased from ATCC, Cat. No. CRL-2577) in a six-well plate, with 500,000 cells per well. Add 2 μL each of DMSO (control group) and avobenzone (experimental group) to the culture wells, add 2000 μL of DMEM complete culture solution to each well, and the final concentration of avobenzone is 10 μM. Mix the culture solution and incubate the treated cells at 37°C for 48 hours.
[0086] 2. Wash the cells twice with PBS,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com