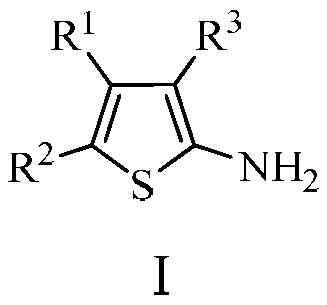
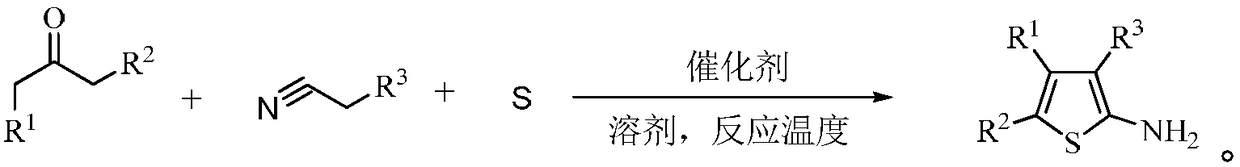
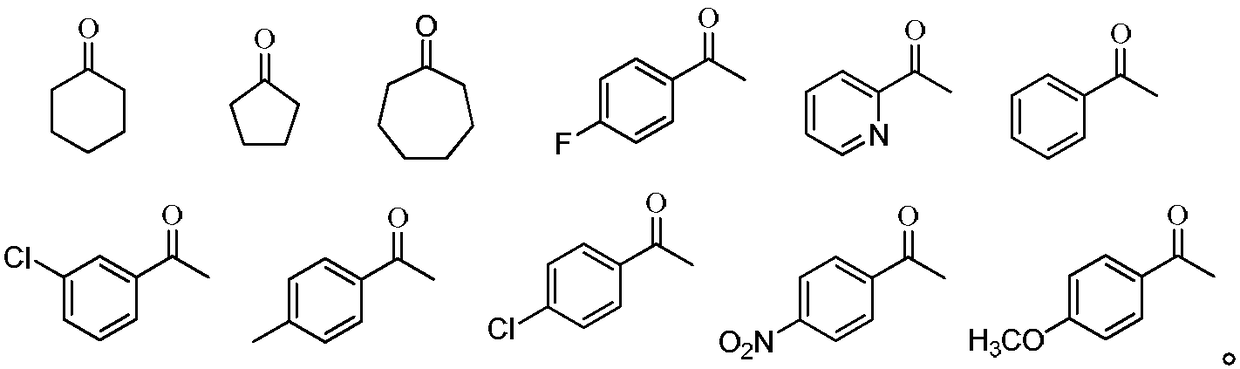
2-aminothiophene compound preparation method
A technology of aminothiophenes and compounds, which is applied in the field of preparation of chemical drug intermediates, can solve the problems of few preparation methods of 2-aminothiophenes, and achieve the effects of strong market competitiveness, simple reaction operation, and safe process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0019] Example 1 Preparation:
[0020] In a 200 mL airtight four-necked reaction flask, add 9.8 g of cyclohexanone, 11.3 g of ethyl cyanoacetate and 3.2 g of sulfur, add 50 mL of solvent toluene and 0.5 mL of catalyst piperidine, stir and control the temperature at 30°C, react for 60 minutes, and complete the reaction , Filtered and recrystallized to obtain 17.5 g of the target product, with a molar yield of 77.8%.
Example Embodiment
[0021] Example 2: Preparation:
[0022] In a 200 mL airtight four-necked reaction flask, add 9.8 g of cyclohexanone, 11.3 g of ethyl cyanoacetate and 3.2 g of sulfur, add 50 mL of solvent methanol and 0.5 mL of catalyst piperidine, stir and control the temperature at 30°C, react for 60 minutes, and complete the reaction , Filtered and recrystallized to obtain 21.7 g of the target product, with a molar yield of 96.4%.
Example Embodiment
[0023] Example 3: Preparation:
[0024] In a 200 mL airtight four-necked reaction flask, add 9.8 g of cyclohexanone, 11.3 g of ethyl cyanoacetate and 3.2 g of sulfur, add 50 mL of solvent methanol and 0.5 mL of catalyst morpholine, stir and control the temperature at 30°C, react for 60 minutes, and complete the reaction , Filtered and recrystallized to obtain 18.6 g of the target product with a molar yield of 93.9%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap