A kind of lentiviral recombinant vector comprising e4bp4 gene and preparation method and application thereof
A technology of lentiviral vectors and recombinant vectors, which is applied in the field of lentiviral recombinant vectors containing E4BP4 gene and its preparation, can solve the problems such as the unclear mechanism of Tfh cells, and achieve the effect of meeting clinical needs and having therapeutic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
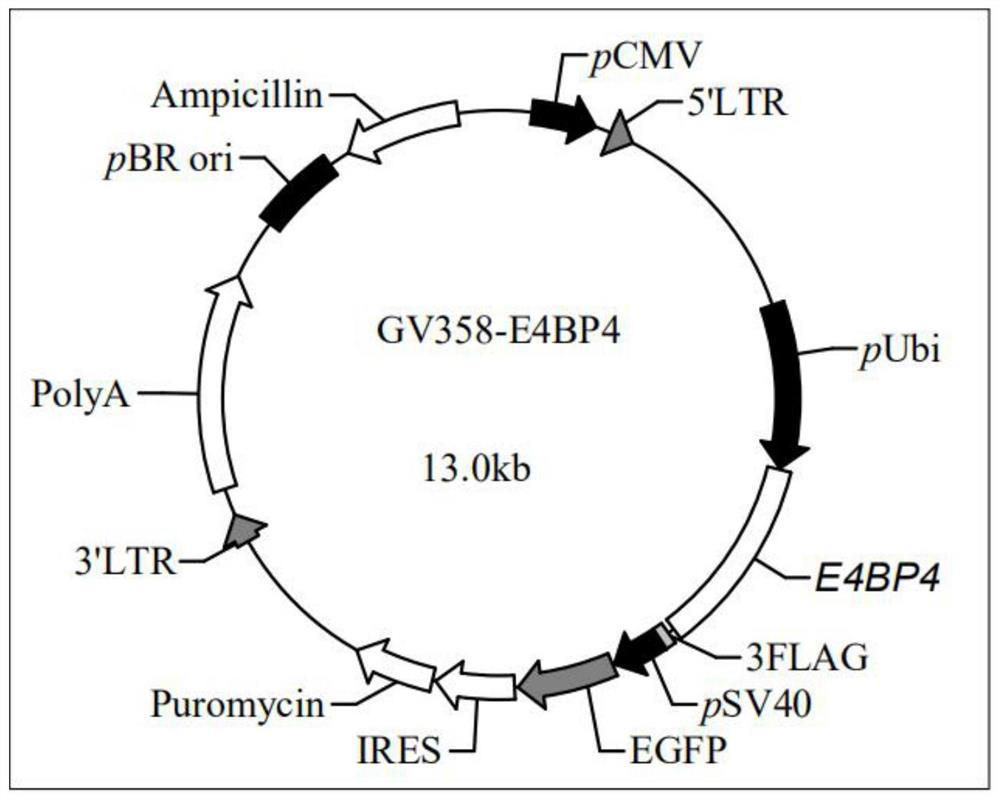
[0029] The present invention also provides a method for preparing the lentiviral recombinant vector containing the E4BP4 gene, comprising the following steps: 1) obtaining the E4BP4 gene fragment by PCR amplification; Double enzyme digestion to obtain the digested E4BP4 gene fragment and the digested GV358 lentiviral vector; 3) Ligate the digested E4BP4 gene fragment described in step 2) and the digested GV358 lentiviral vector to obtain an enzyme containing E4BP4 gene lentiviral recombinant vector.
[0030] In the present invention, when preparing the lentiviral recombinant vector containing the E4BP4 gene, the E4BP4 gene fragment is firstly amplified by PCR. In the present invention, the template of the PCR amplification is the DNA containing the E4BP4 gene, preferably the artificially synthesized DNA whose sequence is shown in SEQ ID No.1; in the specific implementation process of the present invention, the artificially synthesized The DNA whose sequence is shown as SEQID ...
Embodiment 1
[0043] Construction of lentiviral recombinant vector containing E4BP4 gene.
[0044] First synthesize the E4BP4 (as shown in SEQ ID No.1) gene (provided by Shanghai Jikai Gene Co., Ltd.), and then subclone it into: lentiviral vector GV358-Ubi-MCS-3FLAG- SV40-EGFP-IRES-puromycin (provided by Shanghai Jikai Genomics Co., Ltd.) to obtain GV358-Ubi-E4BP4-3FLAG-SV40-EGFP-IRES-puromycin recombinant lentiviral vector. The specific construction process is as follows:
[0045] PCR amplification of E4BP4 gene fragment
[0046] Using the synthesized E4BP4 gene sequence as a template, design the following primers for PCR amplification, so that the two ends of the sequence have AgeI restriction sites and BamH I restriction sites, and the primers are as follows: upstream primer E4BP4-F: gaggatccccgggtaccggtcgccaccatgcagctgagaaaaatgcagac; downstream Primer E4BP4-R: tccttgtagtccatacccccagagtctgaagcagagattg
[0047] The PCR amplification system is shown in Table 1.
[0048] Table 1 PCR amp...
Embodiment 2
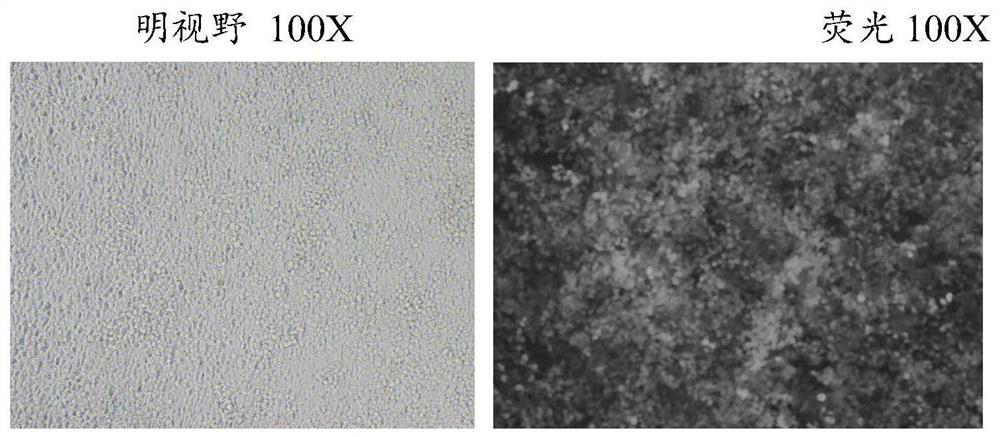
[0076] Lentiviral Particles of Lentiviral Recombinant Vector Containing E4BP4 Gene
[0077] 24 hours before transfection, digest 293T cells in the logarithmic growth phase with trypsin, and adjust the cell density to 5X10 with DMEM high-glucose medium containing 10% FBS fetal bovine serum. 6 Cells / 15ml, seeded in a six-well plate, cultured in a 37°C, 5% CO2 incubator. After 24 hours, the cells can be used for transfection when the cell density reaches 70-80%. The cell state is crucial for virus packaging, so it is necessary to ensure good cell state and less passage times;
[0078] Replace the cell culture medium with serum-free medium 2 hours before transfection;
[0079] Add the prepared DNA solutions (20 μg of lentiviral recombinant vector containing E4BP4 gene, 15 μg of pHelper1.0 vector plasmid, and 10 μg of pHelper2.0 vector plasmid) to a sterilized centrifuge tube, and mix with the corresponding volume of Genechem transfection reagent Evenly, adjust the total volume ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com