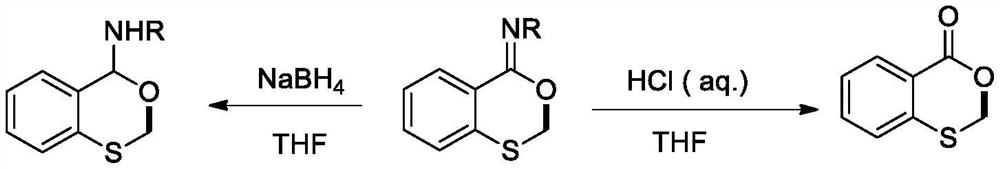
A kind of method for preparing benzo-1,3-oxathiocyclohexadiene-4-ylideneamine
A technology of hexadiene and ylidene amines, applied in the field of preparing benzo-1,3-oxathione-4-ylidene amines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
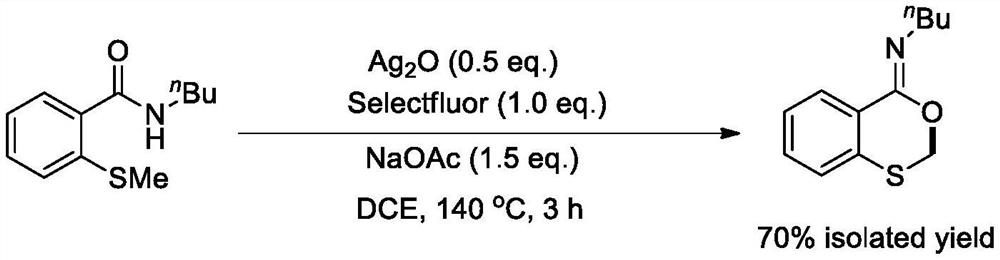
[0015] Take the synthesis method of benzo-1,3-oxathione-4-ylidene n-butylamine as an example: add N-n-butyl-2-methylthiobenzyl one by one into a 100mL pressure-resistant tube Amide (1mmol, 0.22g), sodium acetate (1.5mmol, 0.12g), silver oxide (0.5mmol, 0.12g), 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2 ] Octane bis(tetrafluoroborate) salt (1mmol, 0.35g) and 1,2-dichloroethane (10mL), the reaction temperature was controlled at 140 degrees Celsius, and the reaction was vigorously stirred for 3 hours. After the reaction is finished, cool to room temperature, and then concentrate the reaction solution and separate by column chromatography to obtain benzo-1,3-oxathione-4-ylidene n-butylamine (0.16g, 70% ).
[0016] The equations involved in the reaction are as follows:
[0017]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com