Organic gelling compound of azo phenyl thiourea derivatives, preparation method thereof, organic gel and applications
A technology of azophenylthiourea and organogel, which is applied in the field of supramolecular chemistry, can solve the problems of cumbersome detection methods and processes, and achieve the effect of simple and fast detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The organogel compound of the azophenylthiourea derivative in this example has the structure shown in formula I:
[0045]
[0046] The preparation route of the organogel compound of the azophenylthiourea derivative of this embodiment is as follows:
[0047]
[0048] Concrete preparation method comprises the following steps:
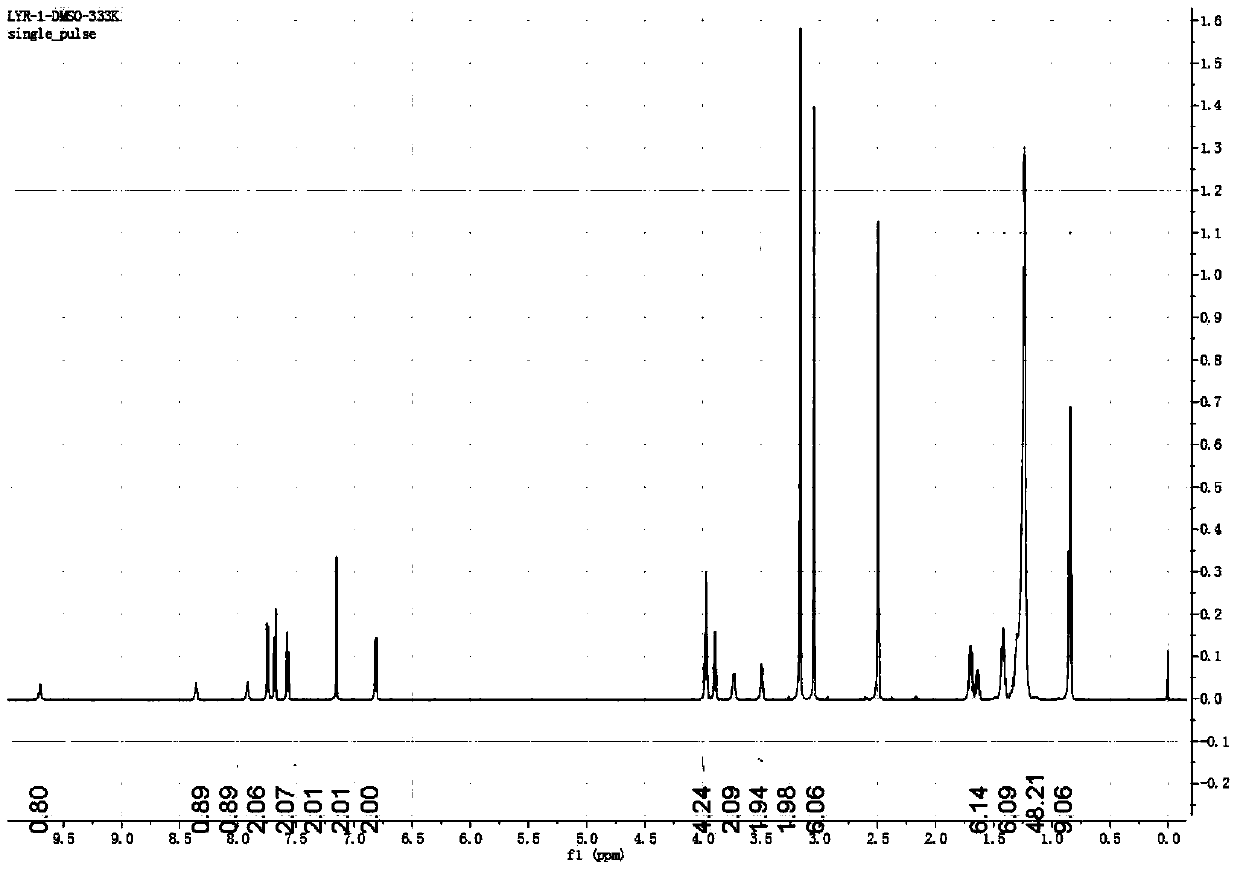
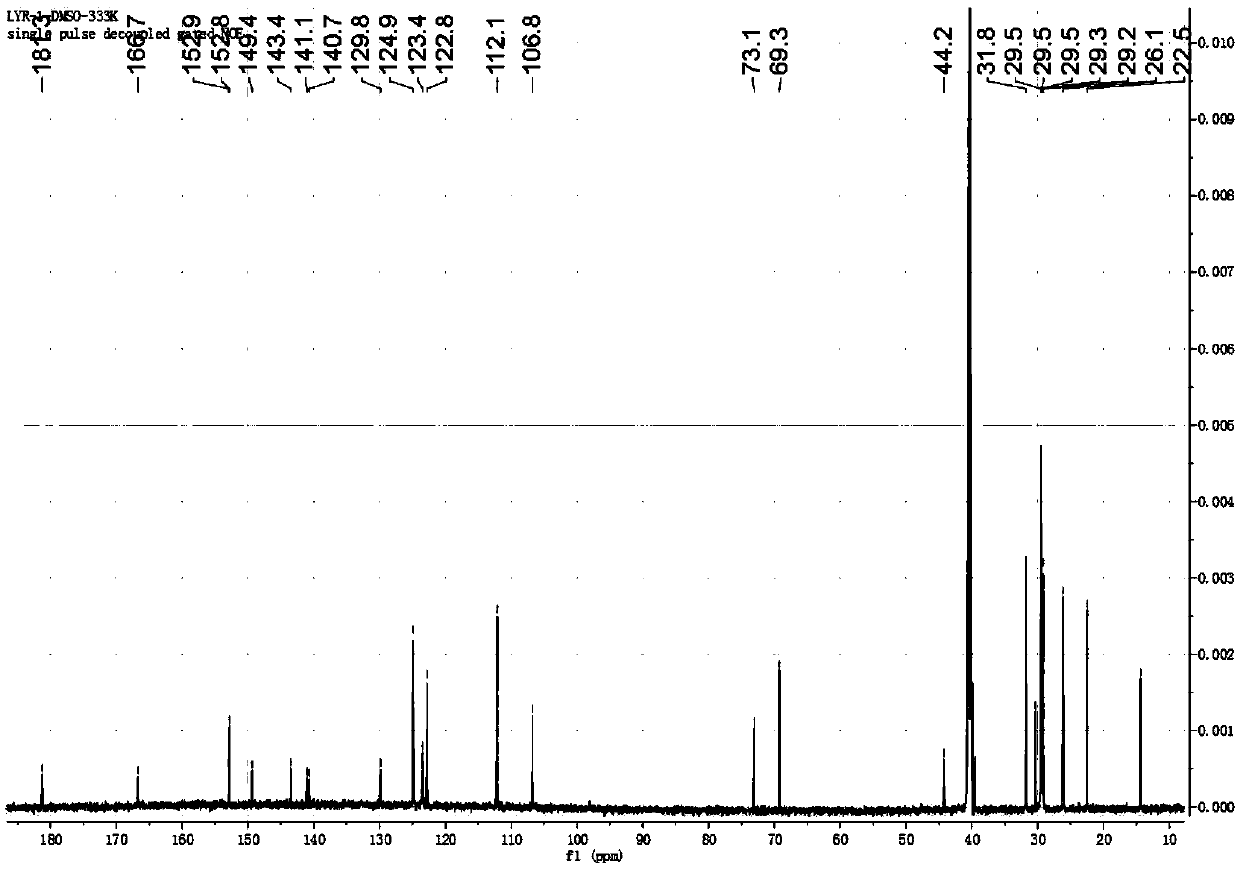
[0049] 1.0g (3.54mmol) 4-dimethylaminoazobenzene-4'-thioisocyanate, 2.54g (3.54mmol) N-(2-aminoethyl)-3,4,5-tri(dodecane Oxy)benzamide was mixed in 100ml of anhydrous tetrahydrofuran, and then refluxed at 90°C for 12h, then the tetrahydrofuran was distilled off under reduced pressure, and the remaining product was purified by a silica gel column, and the eluent was methanol / dichloromethane (1 / 1200, v / v), finally obtained yellow powder, which is the compound shown in formula I, yield 70.5%, 1 HNMR (600MHz, DMSO-d 6 ):9.70(s,1H),8.36(s,1H),7.92(s,1H),7.75(d,J=9.0Hz,2H),7.69(d,J=9.0Hz,2H),7.58(d ,J=9.0Hz,2H),7.15(s,2H),6.82(d,J=9.0Hz,2H),3.99...
Embodiment 2
[0053] The organogel compound of the azophenylthiourea derivative prepared in Example 1 (compound 1 for short) and the organic solvent were placed in a sealed vial, and heated to about the boiling point of the solvent (above 80° C.), so that the azophenylthiourea The organogel compounds of urea derivatives were dissolved and then cooled down to 25°C to observe their gel-forming ability in different solvents. The gels are all thermodynamically reversible, and become flowable sols after heating. The specific gel properties are shown in Table 1.
[0054] The gelation state of organogel compounds of table 1 azophenylthiourea derivatives in different solvents
[0055] Organic solvents
Organic solvents
DMF
S
1,4-dioxane
S
n-Hexane
G(1.9)
G(12.5)
P
G(25.0)
P
P
G(1.8)
S
Dimethyl ...
experiment example 1
[0061] The organogel compound (compound 1) of the azophenylthiourea derivative shown in formula I is to the detection research of metal ion, selects Ag respectively + ,Cd 2+ , Fe 2+ ,K + ,Mg 2+ ,Mn 2+ , Na + , Ni 2+ ,Pb 2+ ,Zn 2+ ,Hg 2+ ,Fe 3+ ,Cu 2+ conduct experiment.
[0062] To the acetonitrile solution of compound 1 (c=10 -4 M) Add different metal ions Ag + ,Cd 2+ ,Fe 3+ ,K + ,Mg 2+ ,Mn 2+ , Na + , Ni 2+ ,Pb 2+ ,Zn 2+ ,Hg 2+ , Fe 3+ ,Cu 2+ ,Such as Figure 5 As shown in a, adding Fe 3+ After the solution turns from yellow to wine red, add Hg 2+ After the solution turned from yellow to bright red, adding Cu 2+ Afterwards, the solution changed from yellow to ginger yellow, and after adding other metal ions, the color had no obvious change; as Figure 5 b shows Fe 3+ and Cu 2+ The acetonitrile solution is colorless, as a control to exclude Fe 3+ and Cu 2+ The influence of the color itself, by Figure 5 It can be seen that compound 1 has an e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com