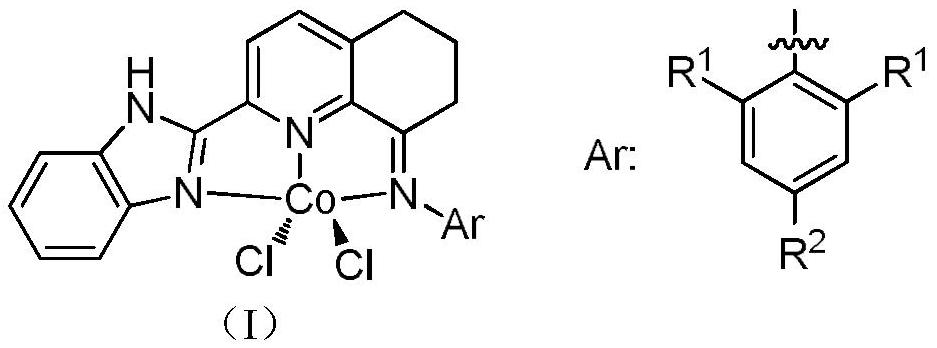
2-Benzimidazolyl-5,6,7,8-tetrahydroquinoline-8-imine cobalt complex and its preparation method and application
A technology based on benzimidazolyl and tetrahydroquinoline, applied in the direction of organic compound/hydride/coordination complex catalysts, hydrocarbons, hydrocarbons, etc., can solve the problems of limited metal catalysts and achieve high catalytic activity , extensive industrial application prospects, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
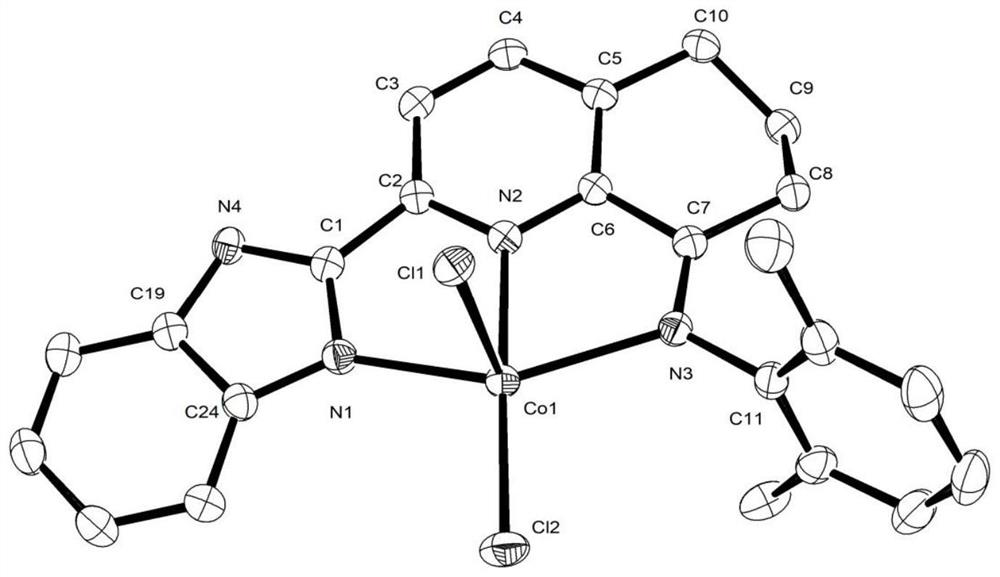
[0047] Example 1: Preparation of 2-benzimidazolyl-5,6,7,8-tetrahydroquinoline-8-imine-(2,6-dimethylanilino) cobalt chloride (II) [coordination Object C1]
[0048]
[0049] Add 158mg 2-benzimidazolyl-5,6,7,8-tetrahydroquinolin-8-one (0.6mmol), 87mg 2,6-dimethylaniline (0.72mmol ) and 74mg anhydrous CoCl 2 (0.57mmol), then add 10mL glacial acetic acid, configure magnetic stirring and spherical condenser, heat and reflux for 8h, produce a large amount of green floc, stop heating, cool down to room temperature, transfer the reaction solution into a one-necked bottle, and pass through the rotary evaporator After concentrating to a small amount of liquid, add 10mL ether to the one-mouth bottle, configure magnetic stirring, and stir at room temperature for 10 minutes to produce a large amount of green flocs. After standing until the flocs sink to the bottom, absorb the upper solvent through the dropper, and repeat This step was performed three times, and finally filtered and dri...
Embodiment 2
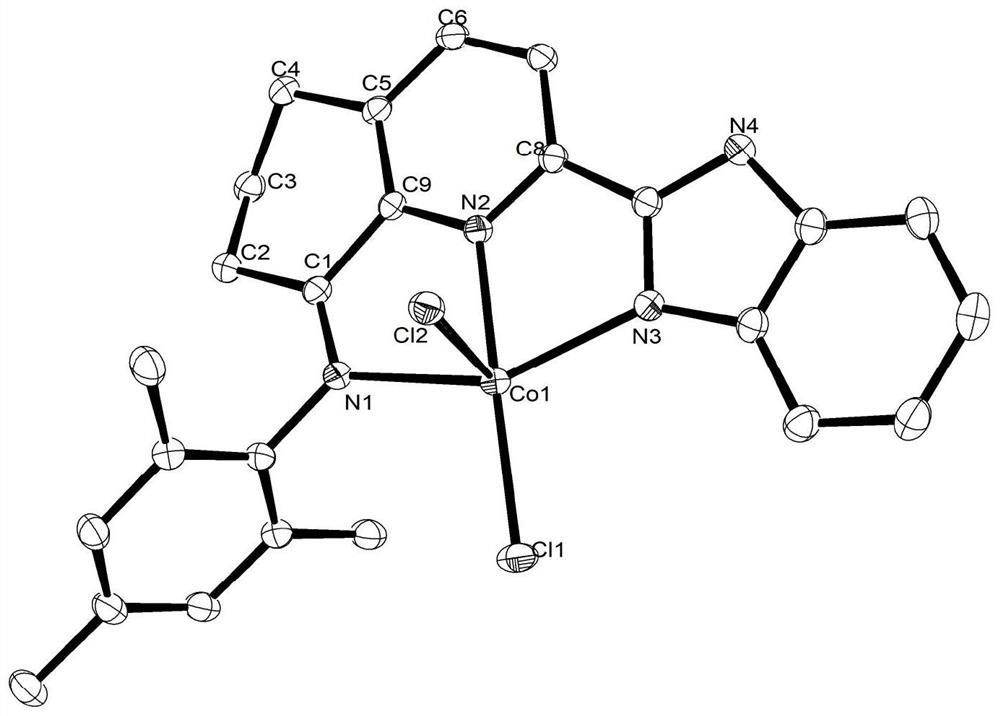
[0051] Embodiment 2: Preparation of 2-benzimidazolyl-5,6,7,8-tetrahydroquinoline-8-imine-(2,6-diethylanilino) cobalt chloride (II) [coordination Object C2]
[0052]
[0053] Add 158mg 2-benzimidazolyl-5,6,7,8-tetrahydroquinolin-8-one (0.6mmol) and 107mg 2,6-diethylaniline (0.72mmol) respectively to a 25mL oblique two-neck flask and 74 mg anhydrous CoCl 2 (0.57mmol), then add 10mL glacial acetic acid, configure magnetic stirring and a spherical condenser, heat and reflux for 8h, a large amount of brown flocs are produced, stop heating, cool down to room temperature, transfer the reaction solution into a single-necked bottle, and pass through a rotary evaporator After concentrating to a small amount of liquid, add 10mL ether to the one-port bottle, configure magnetic stirring, and stir at room temperature for 10 minutes to produce a large amount of brown flocs. After standing until the flocs sink to the bottom, absorb the upper solvent through the dropper, repeat This step ...
Embodiment 3
[0055] Example 3: Preparation of 2-benzimidazolyl-5,6,7,8-tetrahydroquinoline-8-imine-(2,6-diisopropylanilino)cobalt chloride (II)[ Complex C3]
[0056]
[0057] Add 158mg 2-benzimidazolyl-5,6,7,8-tetrahydroquinolin-8-one (0.6mmol), 127mg 2,6-diisopropylaniline (0.72mmol ) and 74mg anhydrous CoCl 2 (0.57mmol), then add 10mL glacial acetic acid, configure magnetic stirring and a spherical condenser, heat and reflux for 8h, a large amount of brown flocs are produced, stop heating, cool down to room temperature, transfer the reaction solution into a single-necked bottle, and pass through a rotary evaporator After concentrating to a small amount of liquid, add 10mL ether to the one-port bottle, configure magnetic stirring, and stir at room temperature for 10 minutes to produce a large amount of brown flocs. After standing until the flocs sink to the bottom, absorb the upper solvent through the dropper, repeat This step was performed three times, and finally filtered and dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com