Method for differential conversion of 7-epitaxol into taxane
A taxane and paclitaxel technology, applied in the field of medicine, can solve the problems of multiple product conversion yields, complicated processes, etc., and achieve the effects of good economic benefits, simple technology, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
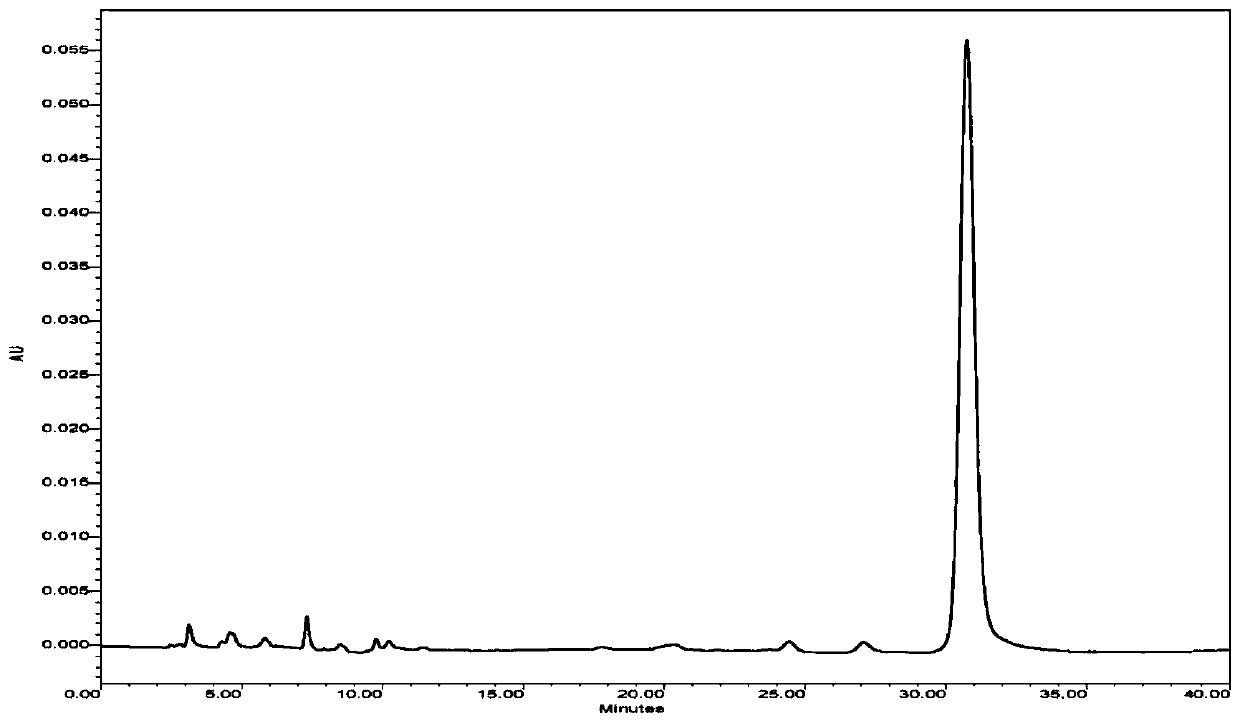
[0026] Embodiment 1: A kind of method of 7-epitaxane epitropic transformation forms taxane, and specific steps are as follows:
[0027] (1) Dissolving 7-epi-paclitaxel in methanol to obtain solution A; wherein the concentration of 7-epi-paclitaxel in solution A is 1 g / L;
[0028] (2) Cobalt chloride is dissolved in the step (1) solution A to obtain the reaction system, and the reaction system is allowed to stand and transform for 72h at a temperature of 0°C to obtain solution B; wherein the concentration of cobalt chloride in the reaction system is 20g / L;
[0029] (3) After step (2) solution B is concentrated and dried in vacuum, carry out column chromatography elution separation to obtain unconverted 7-epitaxol, paclitaxel and cobalt chloride, and unconverted 7-epitaxol and cobalt chloride return Continue conversion in step (2); Wherein eluent is dichloromethane-methanol solvent, and the volume ratio of dichloromethane and methanol in the dichloromethane-methanol solvent is...
Embodiment 2
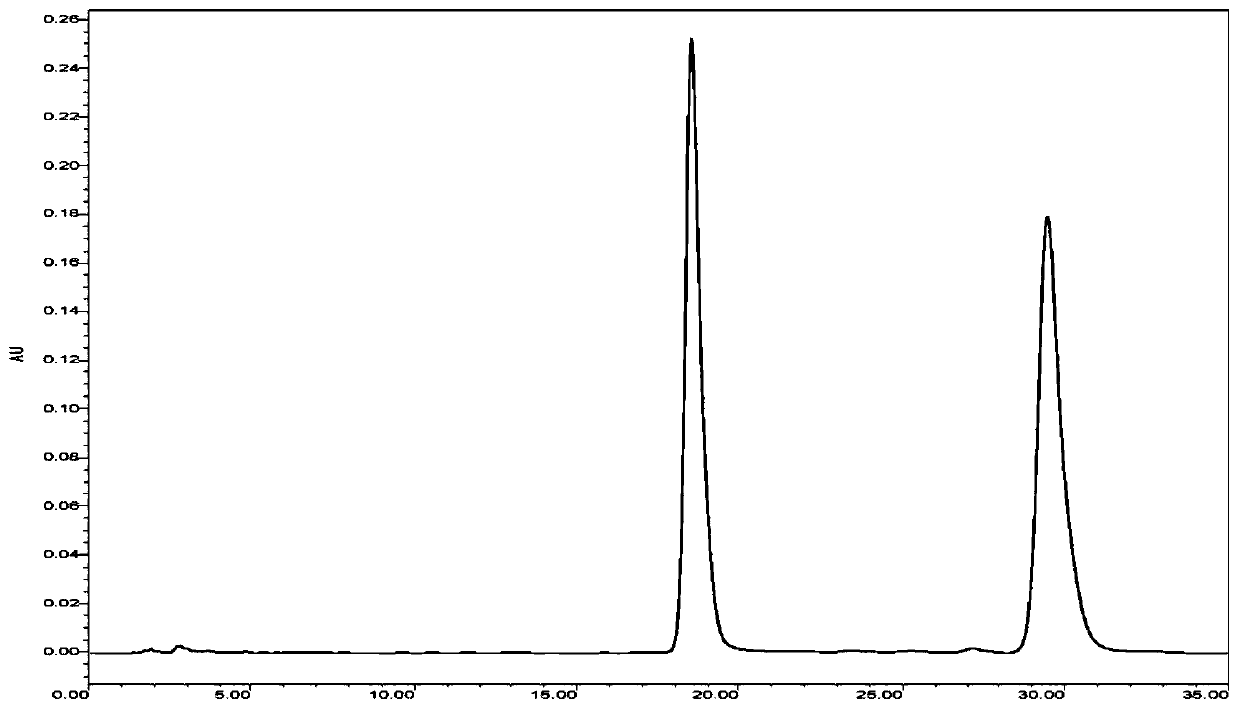
[0032] Example 2: A method for the epi-conversion of 10-deacetyl-7-epi-paclitaxel to form 10-deacetyl-paclitaxel, the specific steps are as follows:
[0033] (1) Dissolving the mixture containing 10-deacetyl-7-epi-paclitaxel in ethanol to obtain solution A; wherein the concentration of 10-deacetyl-7-epi-paclitaxel in solution A is 10 g / L;
[0034] (2) Cobalt chloride is dissolved in the solution A of step (1) to obtain the reaction system, and the reaction system is allowed to stand and transform for 28h at a temperature of 20°C to obtain solution B; wherein the concentration of cobalt chloride in the reaction system is 1g / L;
[0035] (3) Concentrate and dry the solution B of step (2) in vacuo and then carry out column chromatography elution and separation to obtain unconverted 10-deacetyl-7-epitaxol, 10-deacetylpaclitaxel and cobalt chloride, unconverted 10 -deacetylation-7-poor to paclitaxel and cobalt chloride return step (2) to continue conversion; Wherein the eluent is ...
Embodiment 3
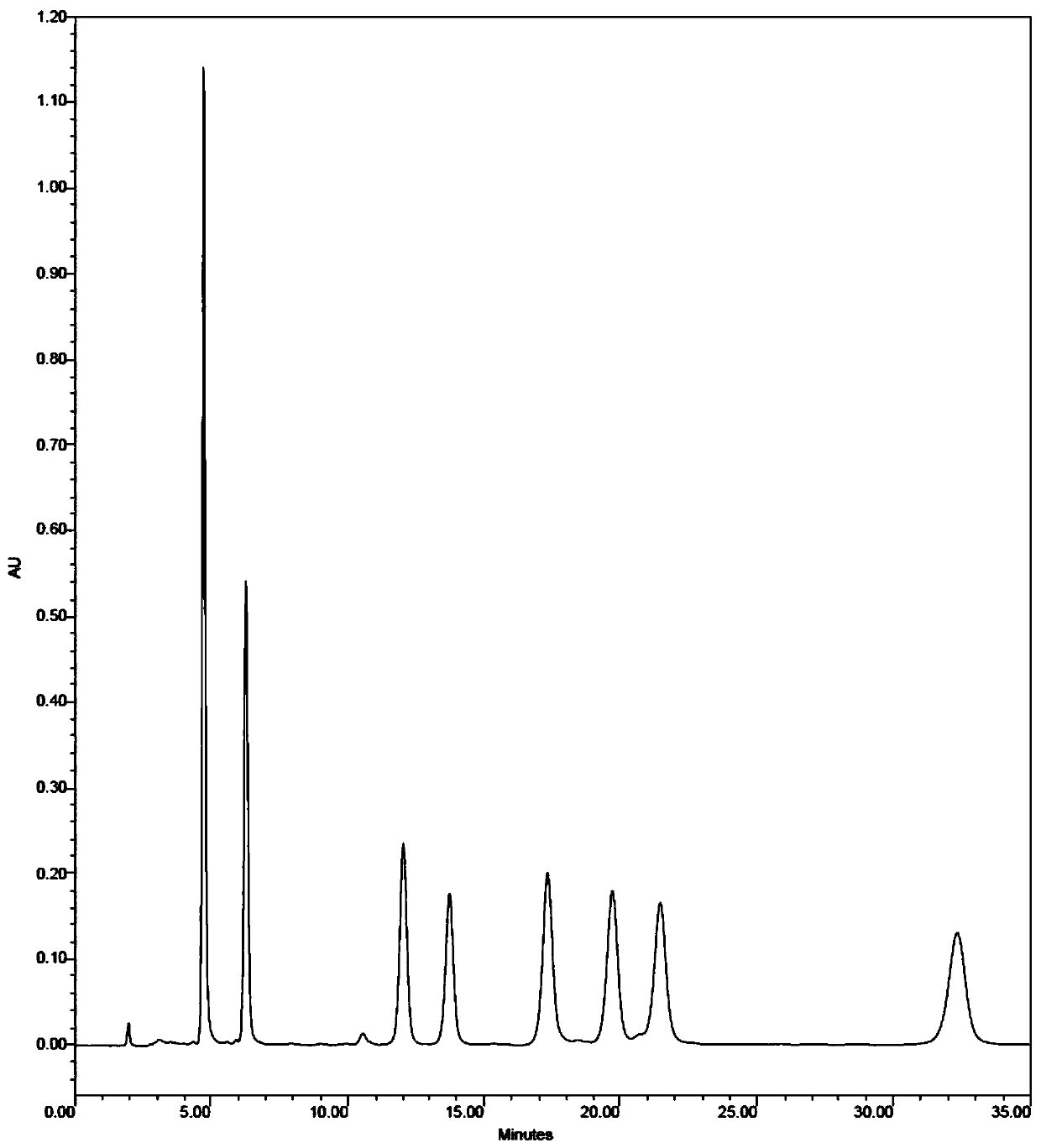
[0038] Embodiment 3: a kind of method of 7- epibaccatin III epitropy conversion forms baccatin III, and concrete steps are as follows:
[0039] (1) Dissolving 7-epibacatin III in dimethyl sulfoxide to obtain solution A; wherein the concentration of 7-epistobacatin III in solution A is 30 g / L;
[0040] (2) cobalt chloride is dissolved in the solution A of step (1) to obtain the reaction system, and the reaction system is left to stand and transformed for 100h under the condition of 50 ℃ to obtain solution B; wherein the concentration of cobalt chloride in the reaction system is 100g / L;
[0041] (3) After step (2) solution B is concentrated and dried in vacuum, carry out column chromatography elution separation to obtain unconverted 7-epiabacatin III baccatin III and cobalt chloride, and unconverted 7-epiabacatin III Cardine III and cobalt chloride return step (2) and leave standstill conversion; Wherein eluent is dichloromethane-methanol solvent, and the volume ratio of dichl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com