Dual-chiral-center cyclopropyl silane compound and preparation and application methods thereof
A technology of cyclopropylsilane and compound, which is applied in the field of chiral center cyclopropylsilane compound and its preparation, can solve the problems of limiting structure diversification and the like, and achieves strong substrate universality, mild reaction conditions and product yield and the effect of high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
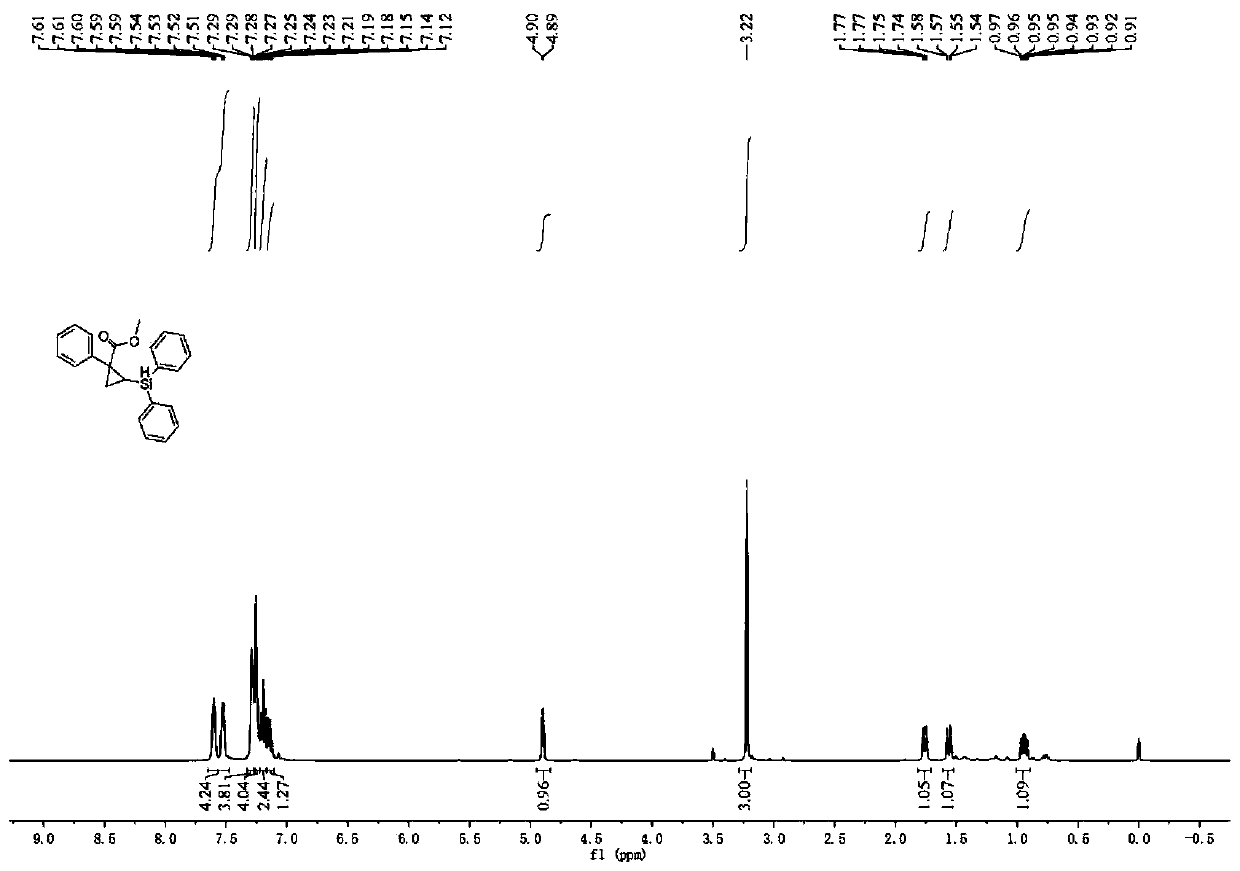
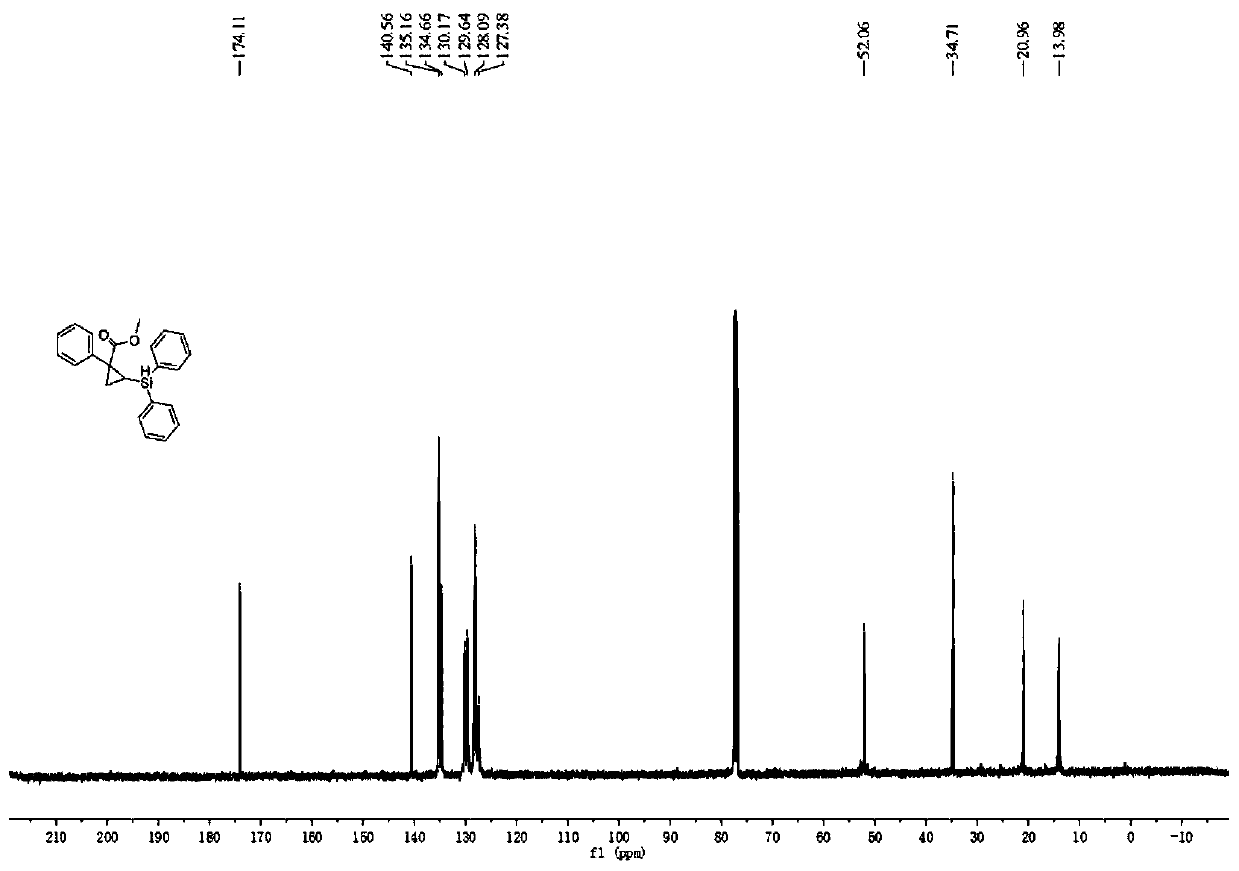
[0045] Under nitrogen atmosphere, add phosphine ligand L2:(R)-DTBM-SegPhos (8.5mg, 0.0072mmol) to Schlenk reaction tube, metal catalyst [Rh(cod) 2 ] BF 4 (2.5mg, 0.006mmol) and additive tetrakis(3,5-bis(trifluoromethyl)phenyl)sodium borate (5.3mg, 0.006mmol), then add 2.0mL n-hexane solvent, and pre-stir at 30°C for 20min Finally, add cyclopropene compound 1a (0.20mmol), stir for 15min, then add diphenylsilane (0.26mmol), continue to stir and react at 30°C for 20h, after the reaction is monitored by TLC, filter, extract, concentrate the filtrate, and pass through silica gel Purified by column chromatography, 50 mg of product 1c was obtained as a light yellow oily liquid with a yield of 70%, 92% ee, 93:7dr.
[0046]
[0047] The physicochemical index of this product: 1 H NMR (400MHz, CDCl 3 )δ7.64–7.57(m,2H),7.53(dd,J=7.1,2.0Hz,2H),7.28(dd,J=6.5,2.6Hz,4H),7.27–7.23(m,4H),7.19 (t,J=7.3Hz,2H),7.17–7.10(m,1H),4.90(d,J=4.4Hz,1H),3.22(s,3H),1.76(dd,J=8.6,3.4Hz, 1H), 1.56(dd,...
Embodiment 2
[0049] Under nitrogen atmosphere, add phosphine ligand L2:(R)-DTBM-SegPhos (8.5mg, 0.0072mmol) to Schlenk reaction tube, metal catalyst [Rh(cod) 2 ] BF 4 (2.5mg, 0.006mmol) and additive tetrakis(3,5-bis(trifluoromethyl)phenyl)sodium borate (5.3mg, 0.006mmol), then add 2.0mL n-hexane solvent, and pre-stir at 30°C for 40min Finally, add cyclopropene compound 1a (0.20mmol), stir for 15min, then add diphenylsilane (0.26mmol), continue to stir and react at 30°C for 20h, after the reaction is monitored by TLC, filter, extract, concentrate the filtrate, and pass through silica gel Purified by column chromatography, 60 mg of product 1c was obtained as light yellow oily liquid with a yield of 84%, 98% ee, 97:3dr.
[0050]
[0051] The physicochemical index of this product: 1 H NMR (400MHz, CDCl 3 )δ7.64–7.57(m,2H),7.53(dd,J=7.1,2.0Hz,2H),7.28(dd,J=6.5,2.6Hz,4H),7.27–7.23(m,4H),7.19 (t,J=7.3Hz,2H),7.17–7.10(m,1H),4.90(d,J=4.4Hz,1H),3.22(s,3H),1.76(dd,J=8.6,3.4Hz, 1H), 1.56(dd, J...
Embodiment 3
[0053] Under nitrogen atmosphere, add phosphine ligand L2:(R)-DTBM-SegPhos (8.5mg, 0.0072mmol) to Schlenk reaction tube, metal catalyst [Rh(cod) 2 ] BF 4 (2.5mg, 0.006mmol) and additive tetrakis(3,5-bis(trifluoromethyl)phenyl)sodium borate (5.3mg, 0.006mmol), then add 2.0mL n-hexane solvent, and pre-stir at 30°C for 60min Finally, add cyclopropene compound 1a (0.20mmol), stir for 15min, then add diphenylsilane (0.26mmol), continue to stir and react at 30°C for 20h, after the reaction is monitored by TLC, filter, extract, concentrate the filtrate, and pass through silica gel Purified by column chromatography, 50 mg of product 1c was obtained as a light yellow oily liquid with a yield of 70%, 90% ee, 90:10dr.
[0054]
[0055] The physicochemical index of this product: 1 H NMR (400MHz, CDCl 3 )δ7.64–7.57(m,2H),7.53(dd,J=7.1,2.0Hz,2H),7.28(dd,J=6.5,2.6Hz,4H),7.27–7.23(m,4H),7.19 (t,J=7.3Hz,2H),7.17–7.10(m,1H),4.90(d,J=4.4Hz,1H),3.22(s,3H),1.76(dd,J=8.6,3.4Hz, 1H), 1.56(dd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com