Bicyclic peptide ligands with detectable moieties and uses thereof
A single-ring, compound technology, applied in the field of peptides, can solve problems such as unsatisfied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0538] Example 1: Peptide Synthesis - Molecular Scaffold Reagents with Leaving Groups
[0539] Peptide synthesis was performed based on Fmoc chemistry using a Symphony peptide synthesizer manufactured by Peptide Instruments and a Syro II synthesizer manufactured by MultiSynTech. Standard Fmoc amino acids (Sigma, Merck) with appropriate side chain protecting groups were employed: where applicable, standard coupling conditions were used in each case, followed by deprotection using standard methods . The peptides were purified using HPLC, and after isolation, they were modified with a molecular scaffold reagent with a leaving group. For this purpose, linear peptides were treated with H 2 O was diluted to about 35 mL, about 500 μL of acetonitrile containing 100 mM molecular scaffold reagent was added, and 5 mL of 1M NH 4 HCO 3 H 2 O initiates the reaction. The reaction was carried out at room temperature for about 30-60 minutes and was lyophilized once the reaction was compl...
example 2
[0540] Example 2: Peptide Synthesis - Molecular Scaffold Reagents Containing Michael Acceptors
[0541] Alternatively, the peptides are purified using HPLC, and after isolation, they are modified with molecular scaffolding reagents containing Michael acceptors. For this, use 50:50MeCN:H 2 O Dilute the linear peptide up to about 35 mL, add about 500 μL of acetonitrile containing 100 mM Michael acceptor-containing molecular scaffold reagent, and wash with 5 mL of 1M NH 4 HCO 3 H 2 O initiates the reaction. The reaction was carried out at room temperature for about 30-60 minutes and was lyophilized once the reaction was complete (as judged by MALDI). Once complete, dissolve 1 mL of 1 M L-cysteine hydrochloride monohydrate (Sigma) in HO at room temperature 2 O was added to the reaction for approximately 60 min to quench any excess molecular scaffold reagents containing Michael acceptors.
[0542] After lyophilization, the modified peptide was purified as described above wh...
example 3
[0544] Example 3: Conjugation of bicyclic peptides to DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid)
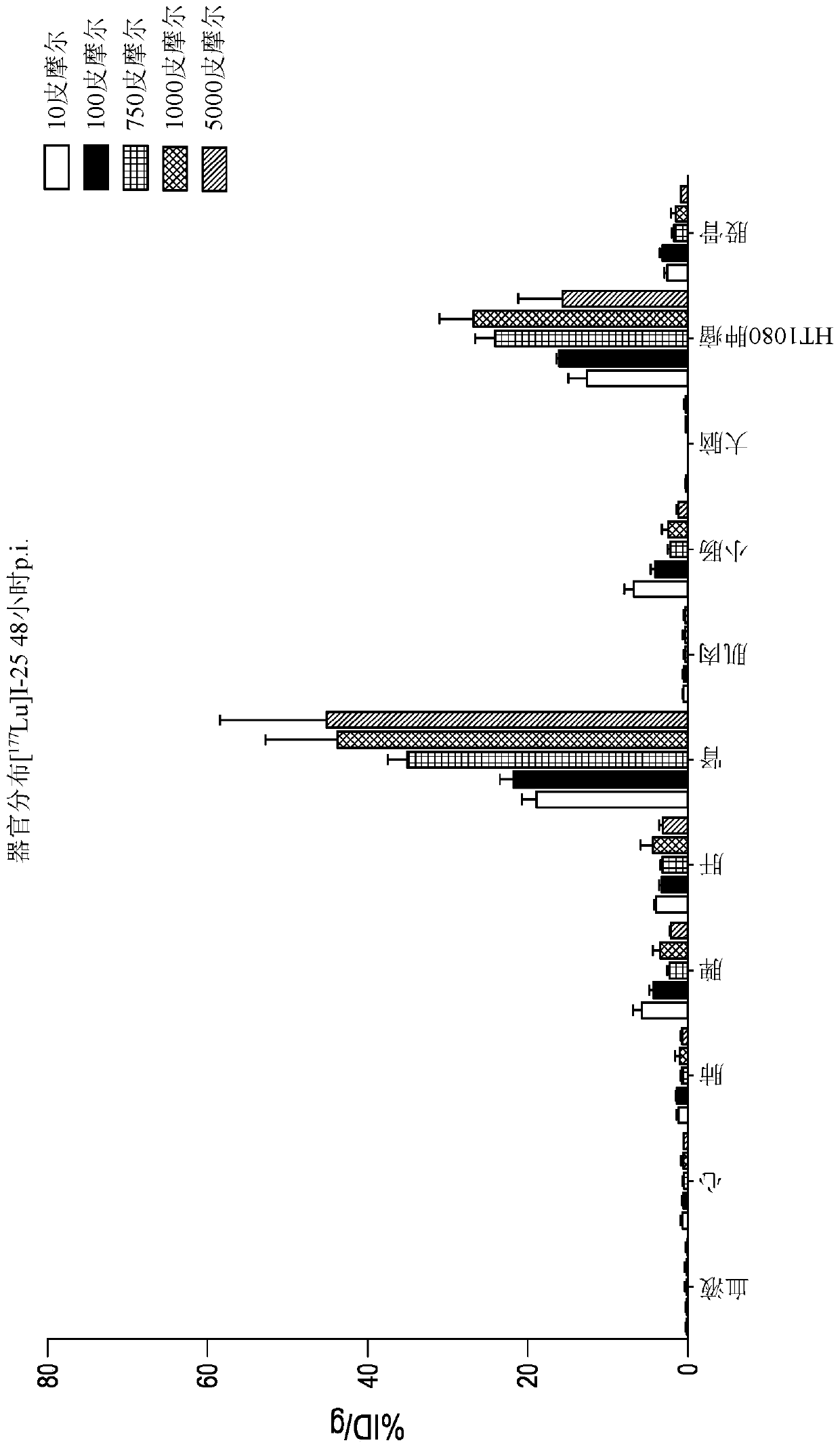
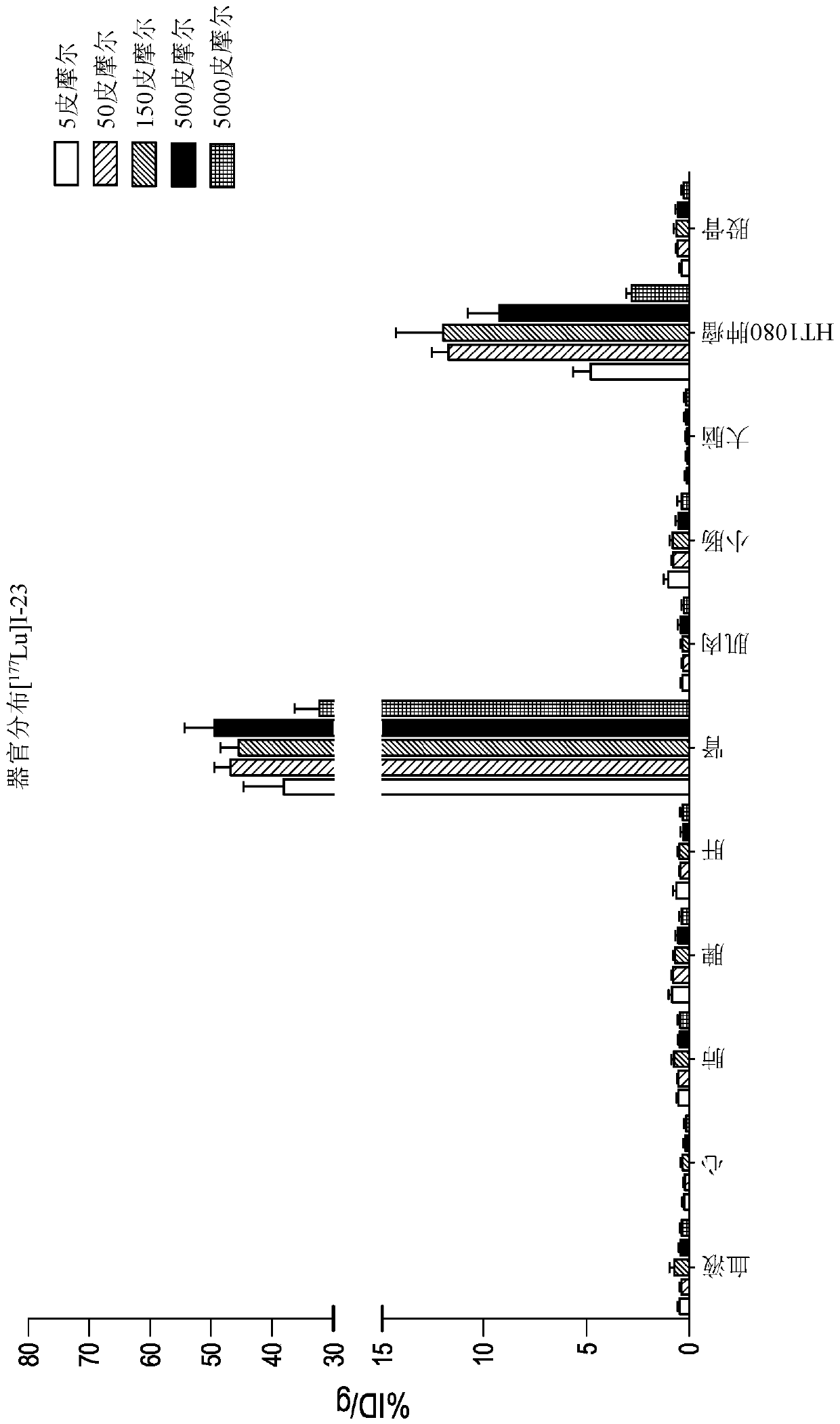
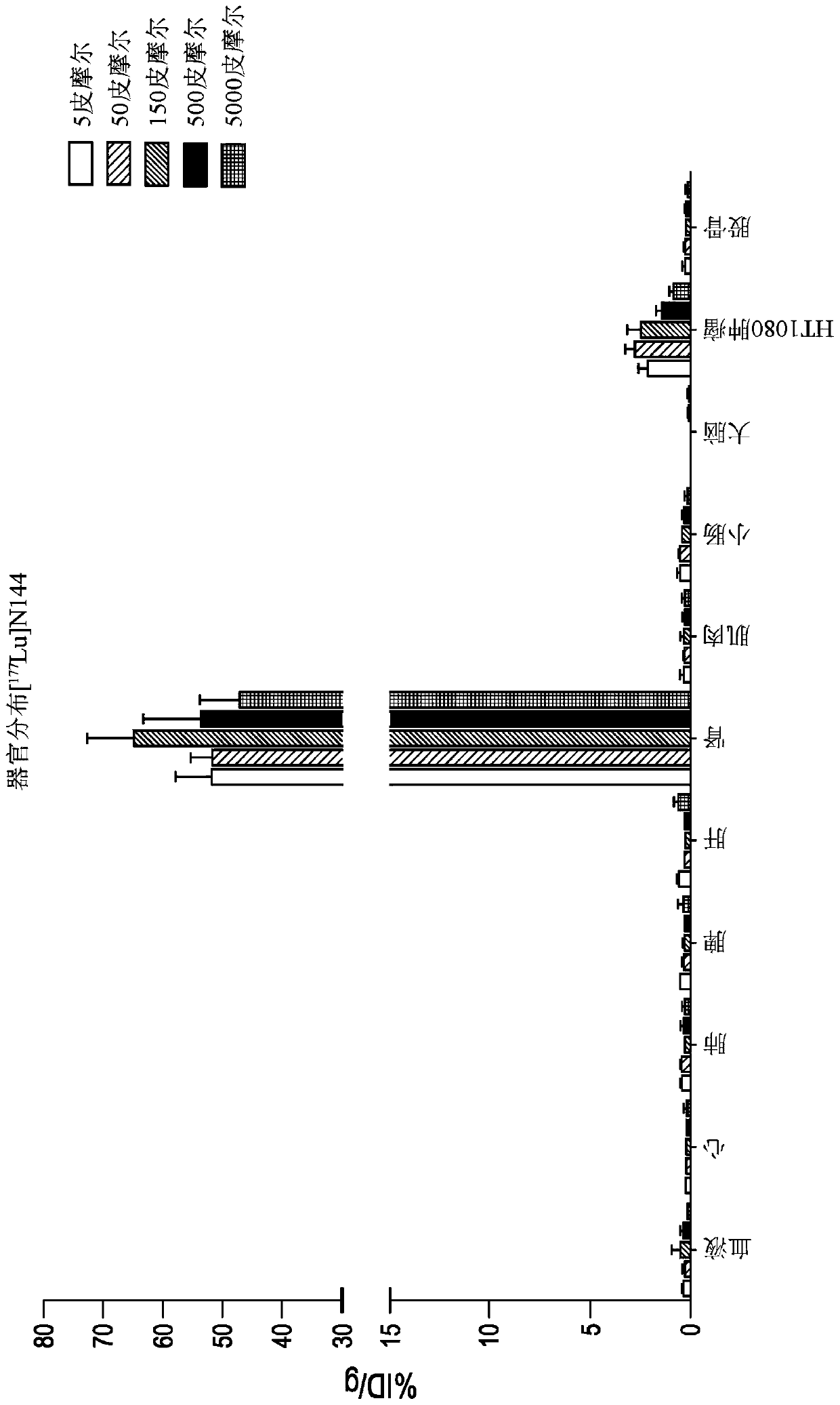
[0545] 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) is a complexing agent that can be conjugated to a bicyclic ring to obtain a construct that The body is using radioactive isotopes (i.e., Lu 3+ ) or MRI contrast agent (ie, Gd 3+ ) labeled for biodistribution studies in animal models. Commercially available DOTA N-succinimide esters are coupled to the amino group of the bicyclic peptide, usually the N-terminal amino group.
[0546]
[0547] name CAS number mw DOTA N-succinimide ester 170908-81-3 761.48
[0548] Materials and methods
[0549] equipment
[0550] LCMS
[0551] MALDI mass spectrometer
[0552] Solvents and compounds and consumables
[0553] Dry dimethyl sulfoxide (DMSO)
[0554] DOTA N-succinimide ester
[0555] N,N-Diisopropylethylamine (DIPEA)
[0556] 100mM Tris(hydroxymethyl)aminometha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com