Preparation method of flaky nano beta-Co(OH)2
A nano and flaky technology, which is applied in the field of preparing flaky nano β-Co2 directly from the cathode cobalt nitrate solution by electrolysis, which can solve the problem of high process conditions and production equipment requirements, difficulty in controlling the particle size of nanoparticles, and difficulty in controlling the concentration of the solution and other problems, to achieve the effect of fewer types, fewer product impurities, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
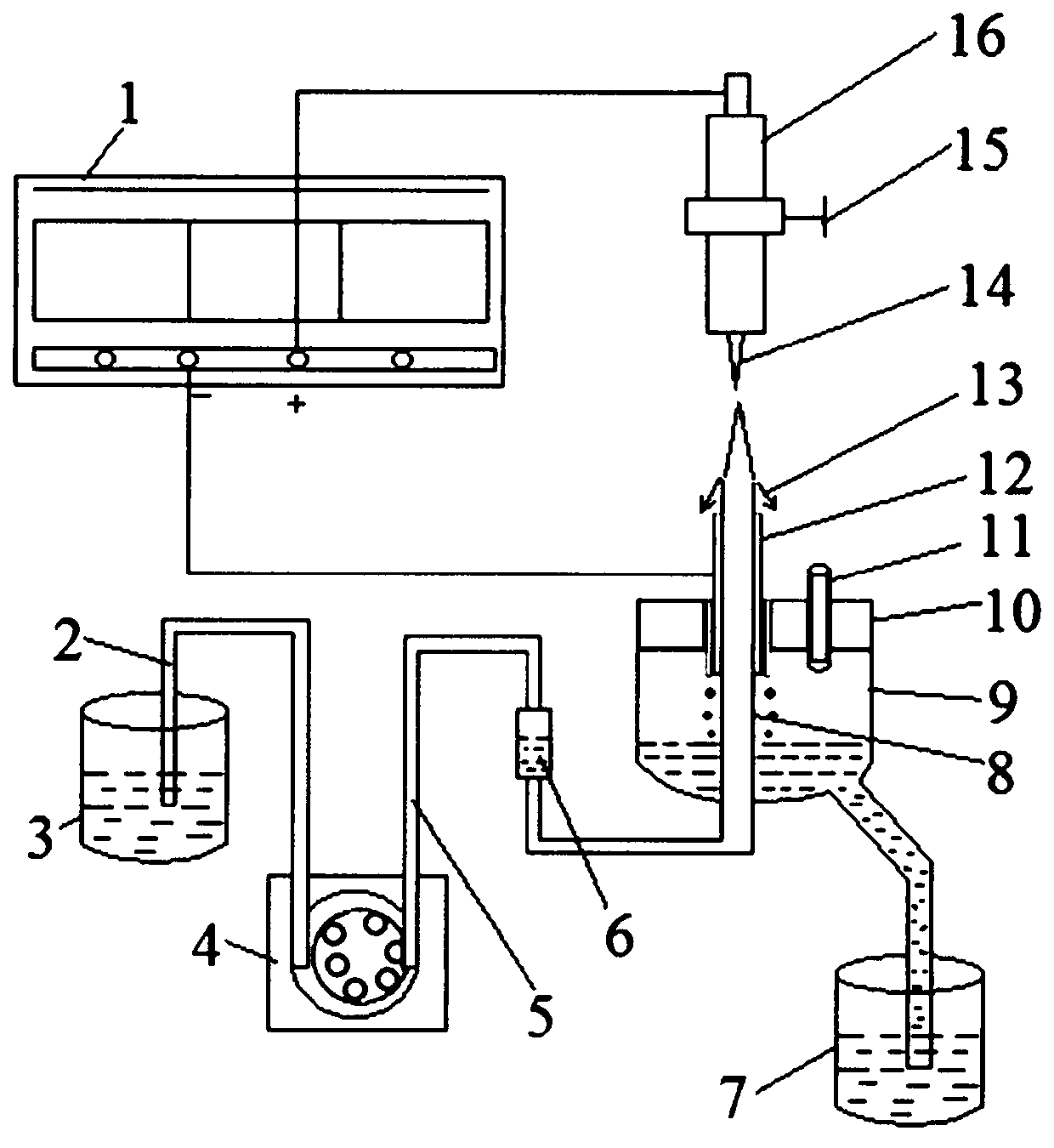
[0034] Electrolyzer structure of the present invention such as figure 1As shown, it includes: solution pool 3, peristaltic pump 4, buffer bottle 6, product pool 7, quartz capillary tube 8, graphite tube 12, liquid collector 9 and platinum needle electrode 14, solution pool 3 is used to add electrolyte, solution pool The first peristaltic pump rubber tube 2 is connected to the inlet end of the peristaltic pump 4, and the outlet end of the peristaltic pump 4 is connected to the 3-7mL buffer bottle 6 through the second peristaltic pump rubber tube 5. The buffer bottle 6 is used to reduce the pressure of the peristaltic pump 4. Pulsation, increasing the stability of electrolysis. Buffer bottle 6 is connected with the lower end of quartz capillary 8, and quartz capillary 8 is vertically arranged, and after the upper end of quartz capillary 8 penetrates liquid collector 9 upwards along the bottom of liquid collector 9, then from the top end cap 10 of liquid collector 9 The bottom o...
Embodiment 2
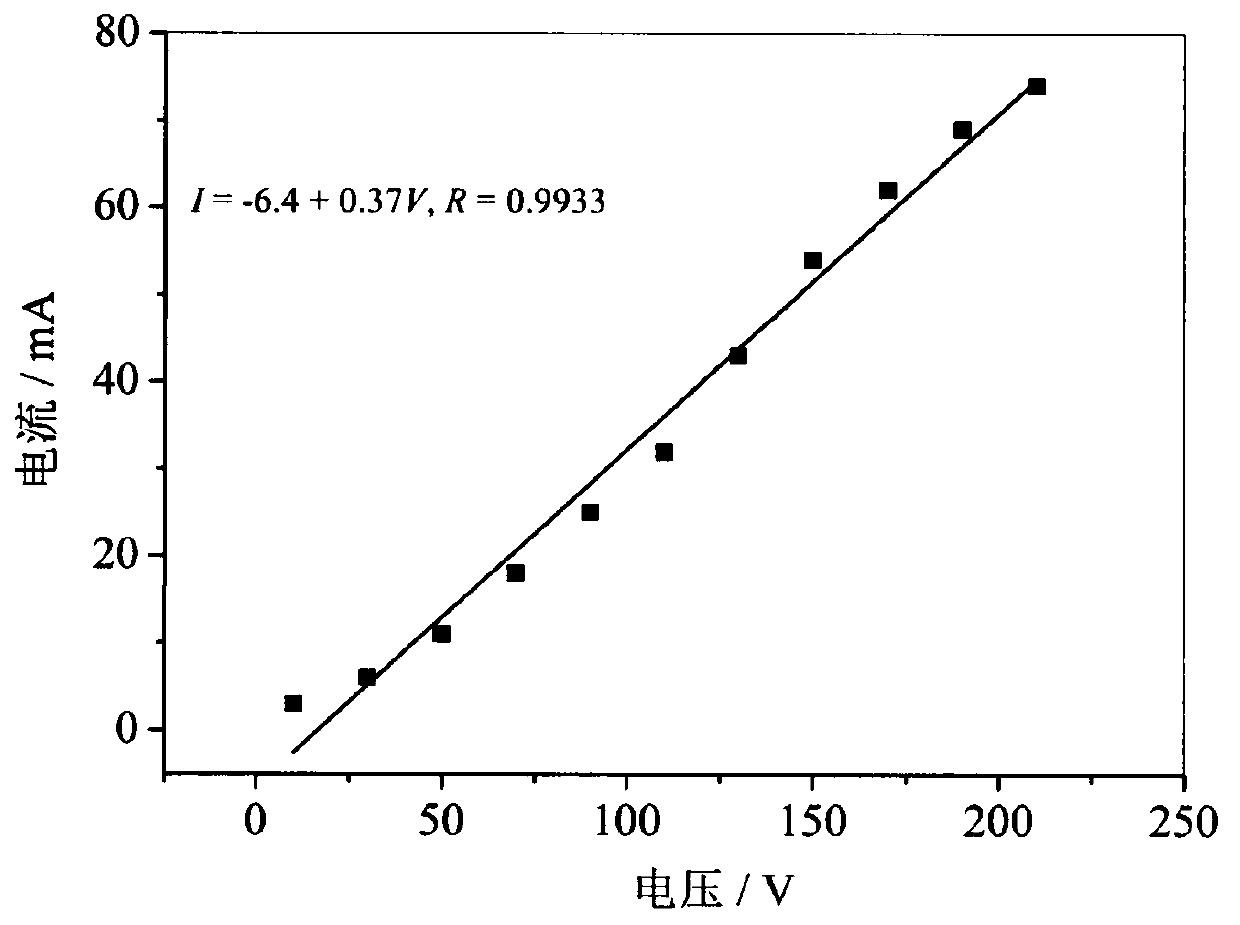
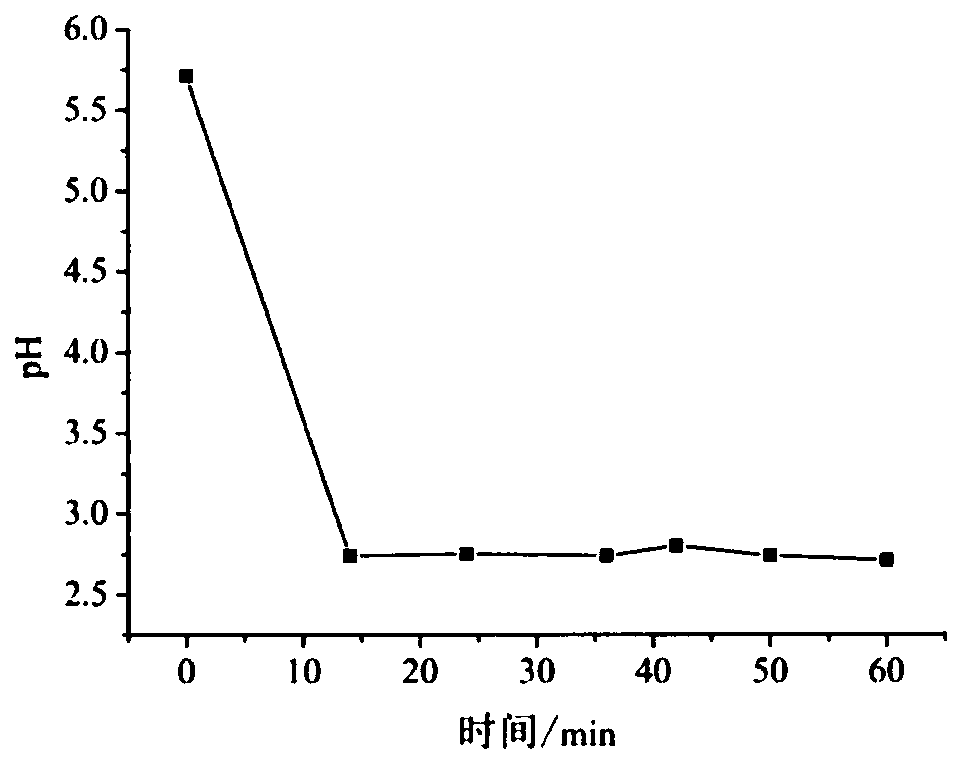
[0036] Flaky Nano β-Co(OH) of the present invention 2 Preparation method: Utilizing the device shown in Example 1, adjust the vertical gap between the upper end of the quartz capillary 8 and the tip of the platinum wire of the platinum needle electrode 14 to be 1.5 mm. Add 0.05mol / L cobalt nitrate solution into the solution pool 3 as the electrolyte, and provide electric energy with a DC power supply. The cobalt nitrate solution flows through the buffer bottle at a flow rate of 2.4mL / min driven by the peristaltic pump, and finally inserts the graphite carbon rod. The top of the capillary overflows, and when a voltage of 150V is applied between the negative and positive poles, the solution overflowing from the top of the capillary turns into a brown turbid liquid after electrolysis, which flows downward along the outer wall of the graphite tube 12 and passes through the gap between the graphite tube 12 and the end cap 10. The continuous gap flows into the liquid collector 9, an...
Embodiment 3
[0038] Flaky Nano β-Co(OH) of the present invention 2 Preparation method: Utilize the device shown in embodiment 1, adjust the vertical gap between the upper end of the quartz capillary 8 and the platinum wire tip of the platinum needle electrode 14 to be 2mm. Add 0.15 mol / L cobalt nitrate solution as electrolyte into the solution pool 3, provide electric energy with a DC power supply, start the peristaltic pump 4, the cobalt nitrate solution flows through the buffer bottle at a flow rate of 3 mL / min driven by the peristaltic pump, and finally insert The top of the capillary of the graphite carbon rod overflows, and when a voltage of 180V is applied between the negative and positive electrodes, the solution overflowing from the top of the capillary becomes a brown turbid liquid after electrolysis, which flows downward along the outer wall of the graphite tube 12 and passes through the graphite tube 12 and the end cap 10 The discontinuous gap between flows into the liquid colle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gap | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap