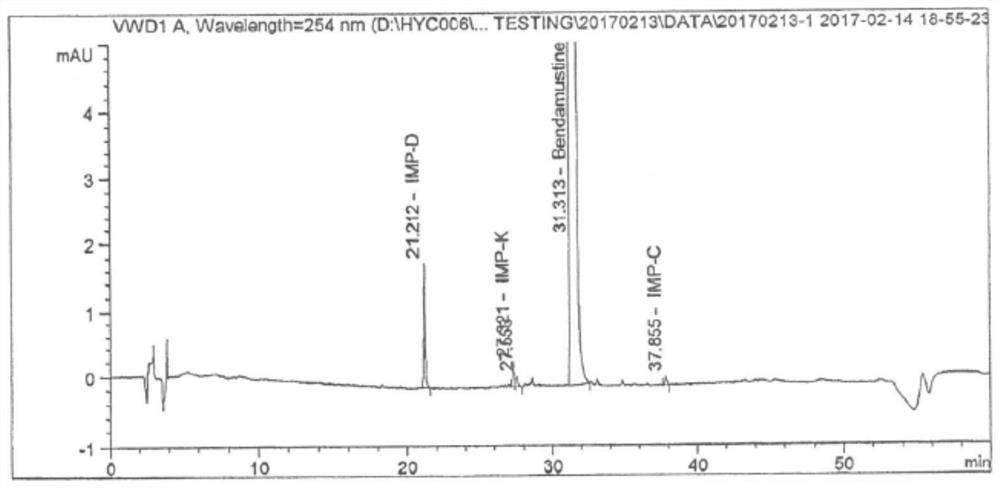
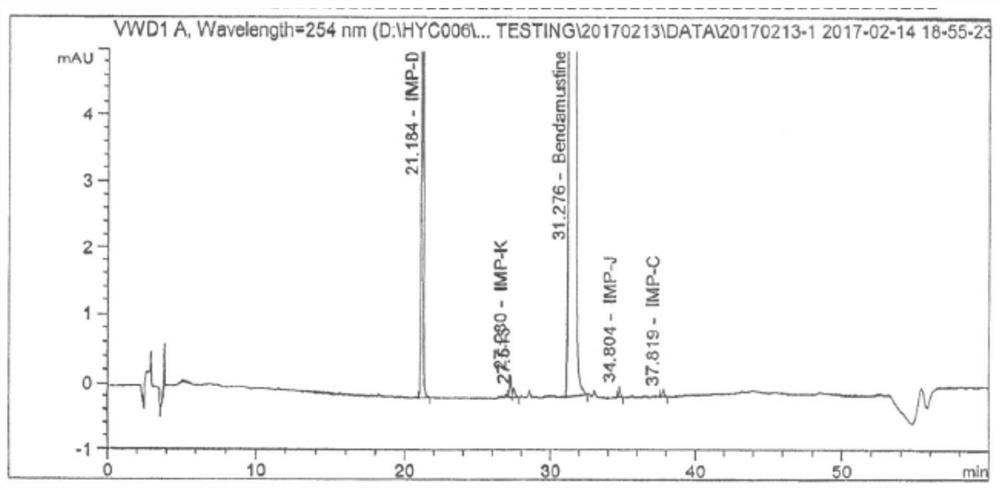
A kind of stable injection bendamustine hydrochloride and preparation method thereof
A technology for bendamustine hydrochloride and injection, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and pharmaceutical formulations, and can solve the problems of low stability of bendamustine hydrochloride and the like , to reduce the difficulty of freeze-drying, improve the stability, and reduce the content of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Prescription information
[0028]
[0029] The stable hydrochloride hydrochloride prepared hydrochloride hydrochloride, including the following steps:
[0030] (1) Add the amount of mannitol in the injection of the injection, stir the complete dissolution, and then add the amount of glacial acetic acid, stir well, and mix well.
[0031] (2) Premimustine, beammustine, which is added to the solution A, stirred completely dissolve, obtain solution B, and dissolve the solution B-filled oxygen to the solution B below 0.5 mg / L, and hold to The filling is over; and the solution B is cooled to 15 ° C;
[0032] (3) Solution B was subjected to sterilization, filling, freeze drying. details as follows:
[0033] The liquid supply can be packed into the pressurization of clean nitrogen, and the solution B is filtered through 2 0.22 μmptfe filter filters to the medium boric silicon glass injection bottle, filling volume 5ml / bottle; holding the dissolved oxygen in solution B before ...
Embodiment 2
[0036] Example 2 had a preparation method for a phenamustine for an injective hydrochloride in Example 1 in:
[0037] The ice acetic acid of Example 2 was 45% (V / V). The prescription information of Example 2 is as follows:
[0038]
Embodiment 3
[0040] The hydrochloride of Example 3 was preparation method for the preparation of the preparation of Example 1 in that the amount of glacial acetic acid in Example 3 was 35% (V / V). The prescription information of Example 3 is as follows:
[0041]
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com