Nitrogen-substituted phenyl pyrrole compound and application thereof in plant sterilization
A technology of phenylpyrroles and compounds, applied in fungicides, plant growth regulators, botanical equipment and methods, etc., can solve the problems of limiting the application range of seed dressings, high synthesis costs, and low bactericidal activity, and achieve improved Select the effect of bactericidal, easy to operate, and high bactericidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
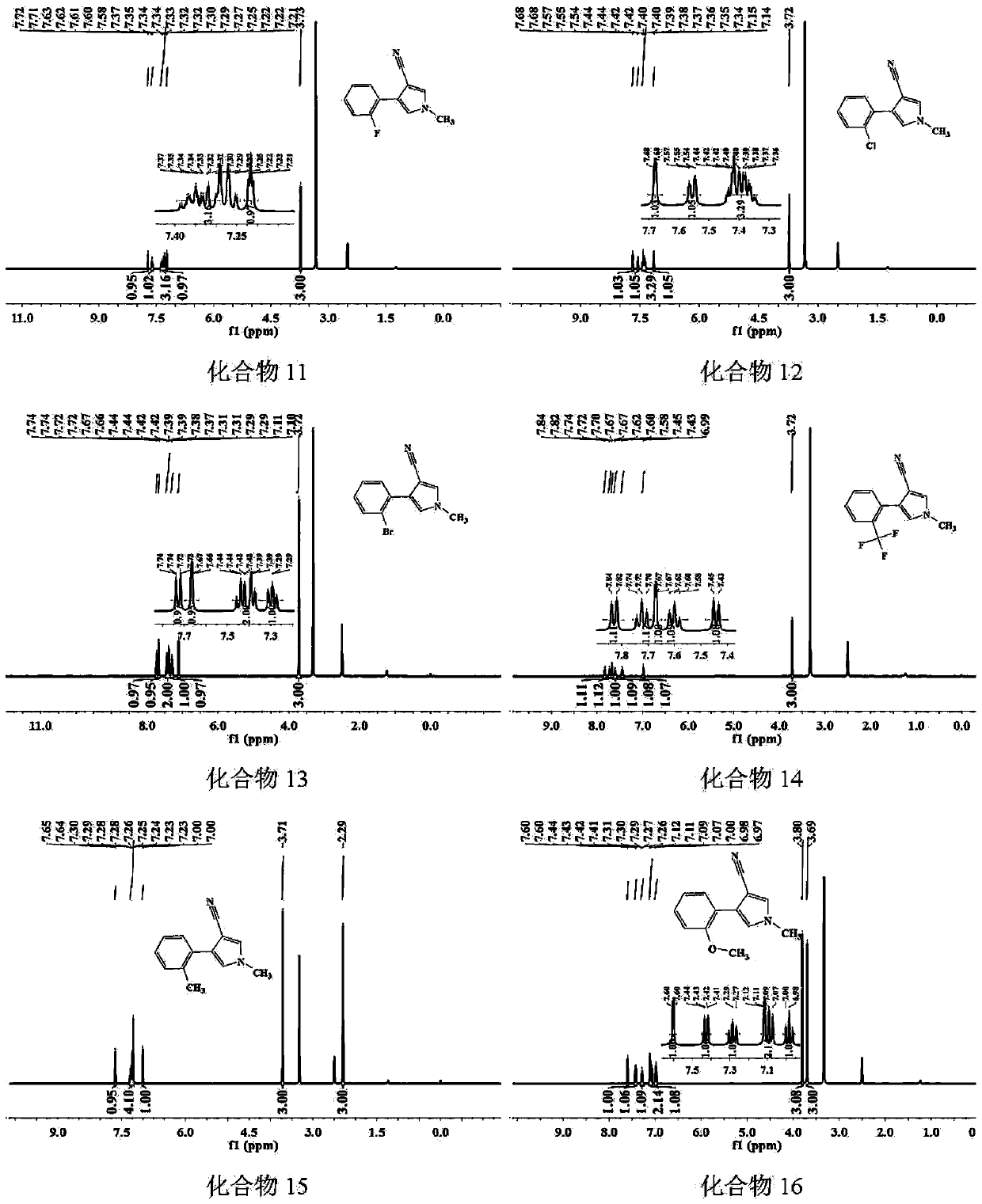
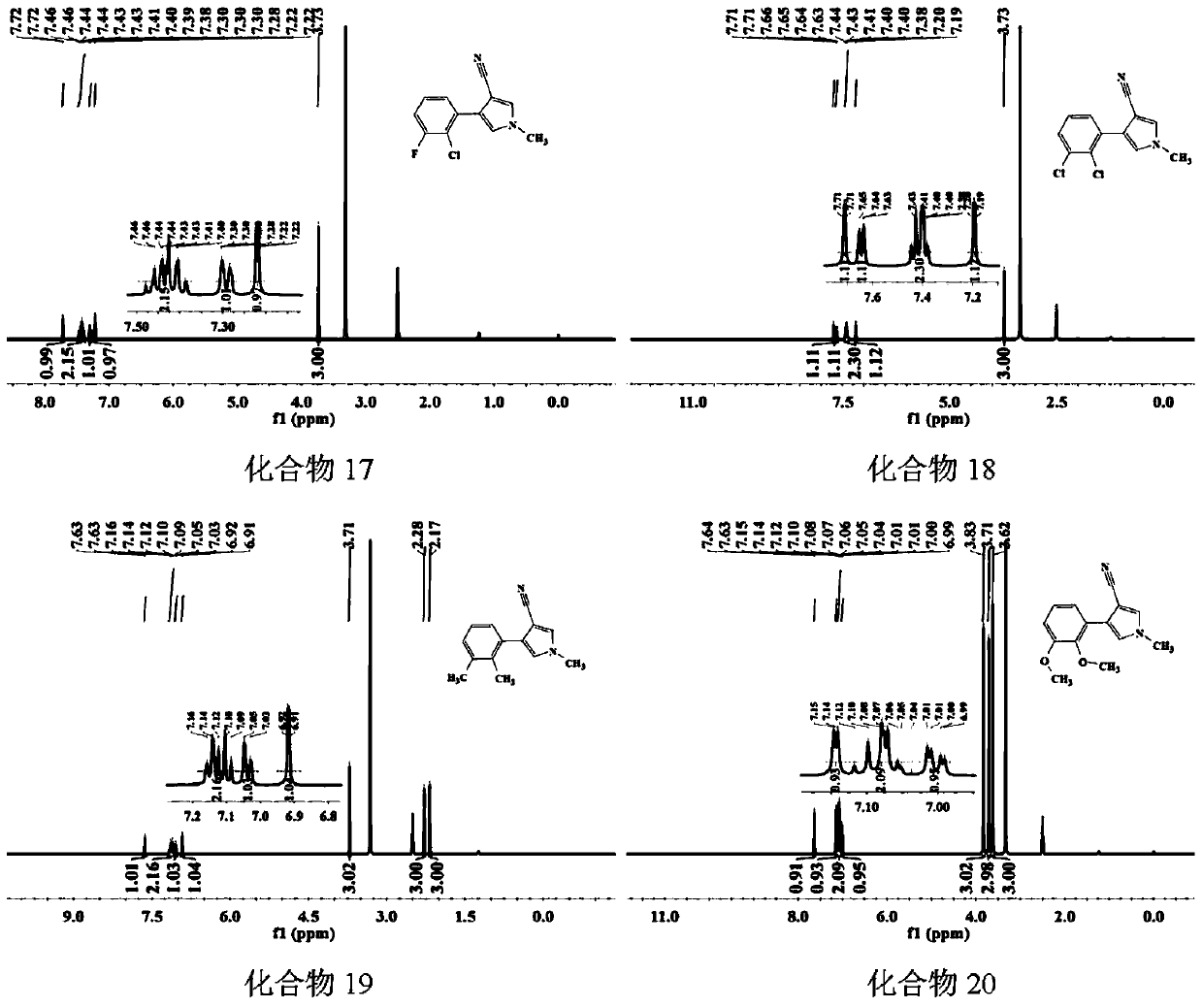
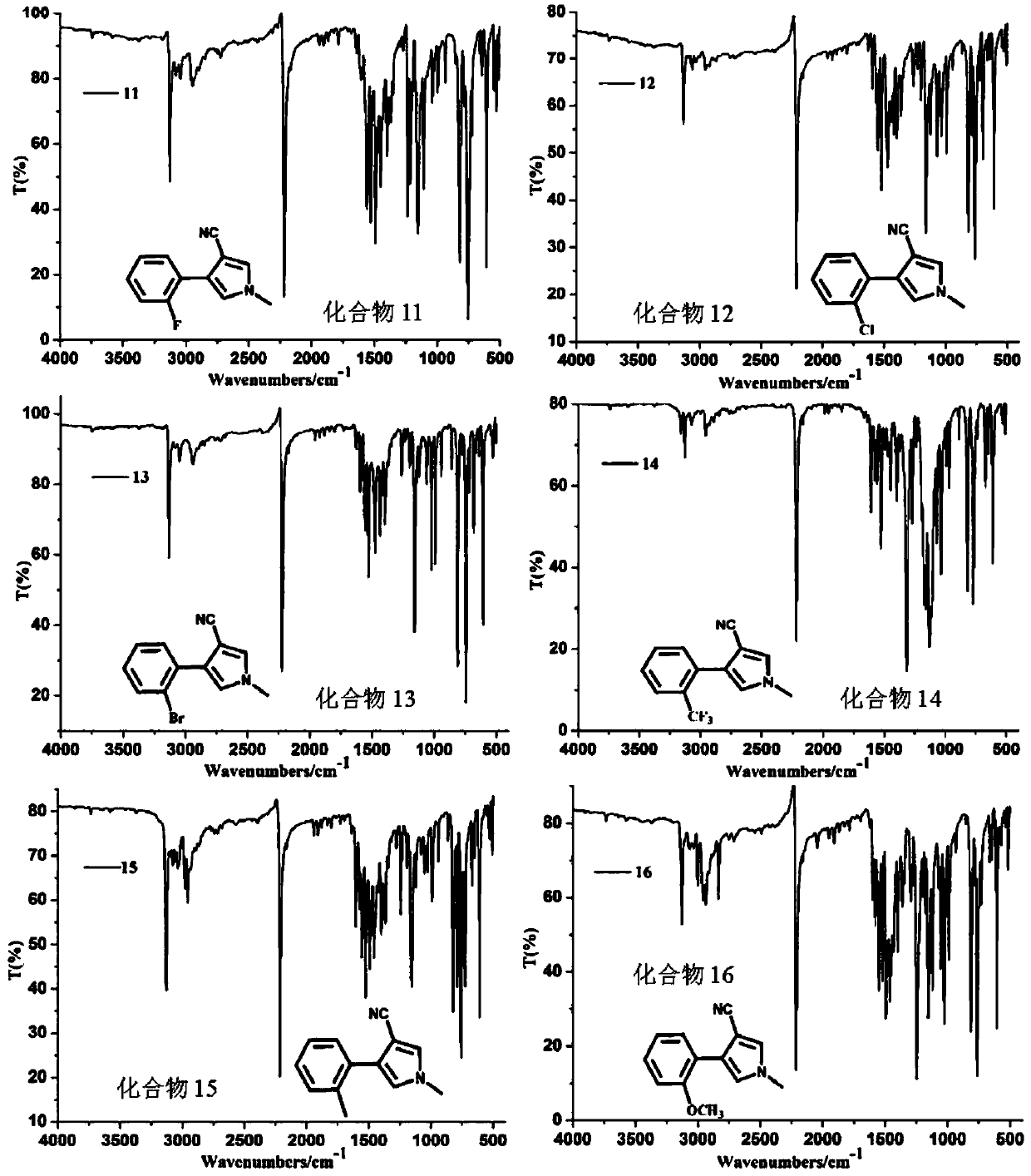
Image
Examples
Embodiment 1
[0153] Add 600g of anhydrous sodium sulfite, 420g of sodium bicarbonate, and 2.4L of water into a four-neck flask equipped with a thermometer, agitator, reflux condenser, and dropping funnel, raise the temperature to 70-80°C, and then add p-toluene in batches Sulfonyl chloride 484g, add 5~10g every batch, keep the reaction temperature of temperature 80 ℃ and continue to react for 1 hour. Cooled with ice water, left for 1 hour, and filtered to obtain 434g of sodium p-toluenesulfinate with a purity of 99% and a yield of 96%.
[0154] Add dry sodium p-toluenesulfonate 428g, paraformaldehyde 279.84g, formamide 790.6g and 540.4g of anhydrous formic acid, heated to 90°C for 2 hours, then cooled to room temperature, added 1750mL of water, cooled with ice water to cool down to precipitate a white solid, continued to stir for 1.5 hours, filtered, washed, and dried to obtain p-toluenesulfonylmethyl Formamide 387.7g, purity 98%, yield 78.1%.
[0155] Add 42.6 g of p-toluenesulfonylmethyl...
Embodiment 2
[0160] In a 250mL four-necked flask equipped with a thermometer, a stirrer, and a reflux condenser, 80mL of ethanol was charged, 7.0g of 2-chlorobenzaldehyde, 4.62g of cyanoacetamide and 4.23g of ammonium acetate were added, and 0.5g of TEBA was heated to 85 ~90°C, reflux reaction for 2 hours, then add 100mL deionized water and stir for 30 minutes, precipitate and filter to obtain 14.2g of 2-cyano-3-(2-fluorophenyl)acrylamide, purity 97.2%, yield 67.9%, product melting point It is 167.5-168°C.
[0161] Put 50mL of methanol and 12.5mL of dichloromethane into a 250mL four-neck flask equipped with a thermometer, a stirrer, a reflux condenser, and a dropping funnel, and add 2-cyano-3-(2-fluorophenyl)acrylamide 10.3g and 9.75g p-toluenesulfonylmethyl isonitrile (TosMIC), add dropwise a mixed solution composed of 20mL methanol and 5.6g KOH under the condition of ice bath, react at room temperature for 3h, evaporate the solvent under reduced pressure, add 50 mL of deionized water wa...
Embodiment 3
[0164] Put 80mL of ethanol in a 250mL four-necked flask equipped with a thermometer, a stirrer, and a reflux condenser, add 18.5g of 2-bromobenzaldehyde, 9.24g of cyanoacetamide and 11.1g of triethylamine, and heat to 85-90°C. Reflux for 2 hours, then add 100 mL of deionized water and stir for 30 minutes, precipitate and filter to obtain 15.73 g of 2-cyano-3-(2-bromophenyl)acrylamide, with a purity of 97.5%, a yield of 62.7%, and a melting point of 130.0°C.
[0165] Put 50mL methanol and 12.5mL methylene chloride in a 250mL four-neck flask equipped with a thermometer, a stirrer, a reflux condenser, and a dropping funnel, and add the 2-cyano-3-(2-bromophenyl ) 3.95g of acrylamide and 3.1g of p-toluenesulfonylmethyl isonitrile (TosMIC), dropwise added a mixture of 20mL of methanol and 1.79g of KOH in an ice bath, reacted at room temperature for 3h and evaporated under reduced pressure Solvent, add 50mL deionized water and stir for 0.5h, filter and dry, and recrystallize with met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com