Tablets for treating vascular hypertension and production method of tablets for treating vascular hypertension
A technology for hypertension and tablets, which is applied in the field of tablets for the treatment of hypertension and its preparation, can solve the problems of difficult industrial production, complicated process, and low dissolution rate of tablets, and achieve fast drug dissolution speed and preparation The effect of simple process and rapid disintegration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0037] Example 1
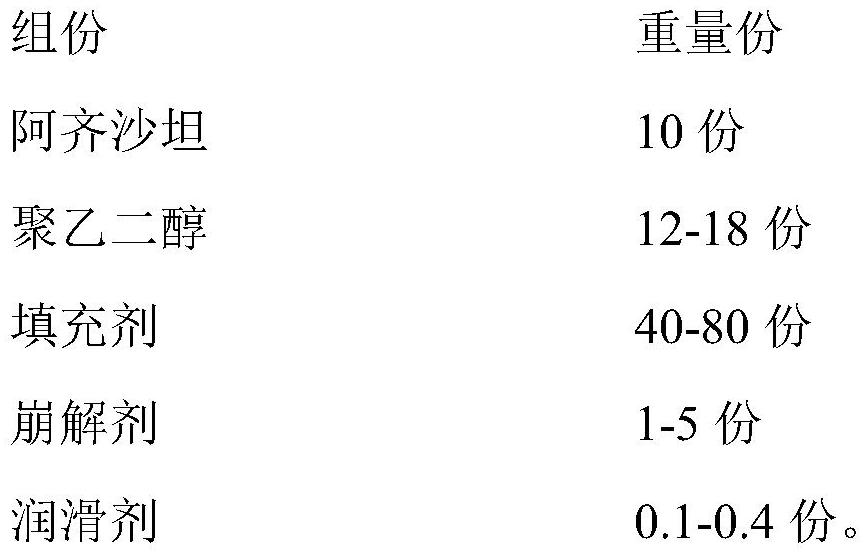
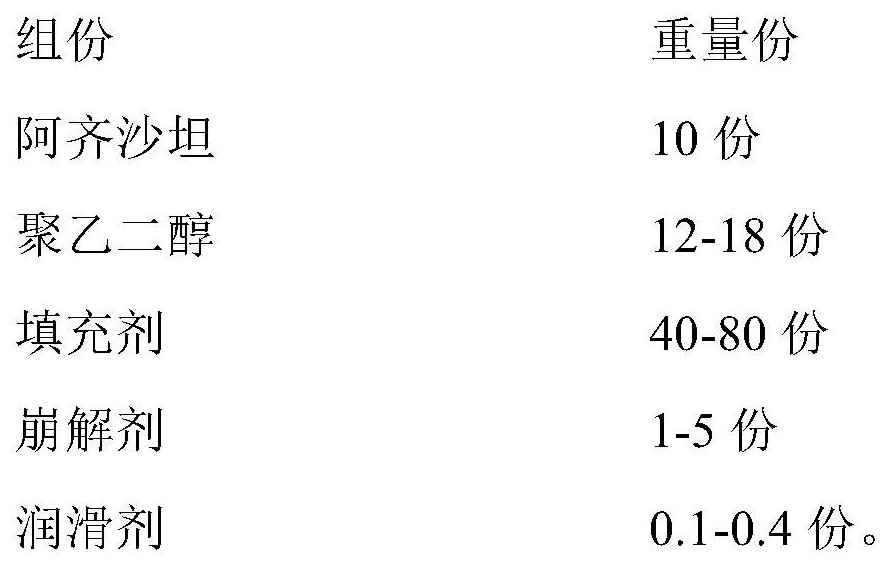
[0038] 1) Prescription
[0039]
[0040] 2) Preparation method
[0041] Azilsartan was added to the melt of polyethylene glycol 4000 and PEG1500, stirred evenly to obtain a suspension melt;
[0042] Weigh the prescribed amount of corn starch, lactose, and low-substituted hydroxypropyl cellulose and mix them evenly, add the above-mentioned suspension melt, stir and granulate, add magnesium stearate to the prepared granules, mix, and compress.
Example Embodiment
[0043] Example 2
[0044] 1) Prescription
[0045]
[0046]
[0047] 2) Preparation method
[0048] Azilsartan was added to the polyethylene glycol 2000 melt and stirred evenly to obtain a suspension melt;
[0049] Weigh the prescribed amount of corn starch, lactose, and low-substituted hydroxypropyl cellulose and mix them evenly, add the above-mentioned suspension melt, stir and granulate, add magnesium stearate to the prepared granules, mix, and compress.
Example Embodiment
[0050] Example 3
[0051] 1) Prescription
[0052]
[0053] 2) Preparation method
[0054] Azilsartan was added to the polyethylene glycol 6000 melt and stirred evenly to obtain a suspension melt;
[0055] Weigh the prescribed amount of corn starch, lactose, and low-substituted hydroxypropyl cellulose and mix them evenly, add the above-mentioned suspension melt, stir and granulate, add magnesium stearate to the prepared granules, mix, and compress.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap