Glycyrrhetinic acid clathrate compound and preparation method thereof
A technology of glycyrrhetinic acid and inclusion compound, applied in the field of glycyrrhetinic acid inclusion compound and its preparation, to achieve the effects of improving embedding rate, mild preparation conditions and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
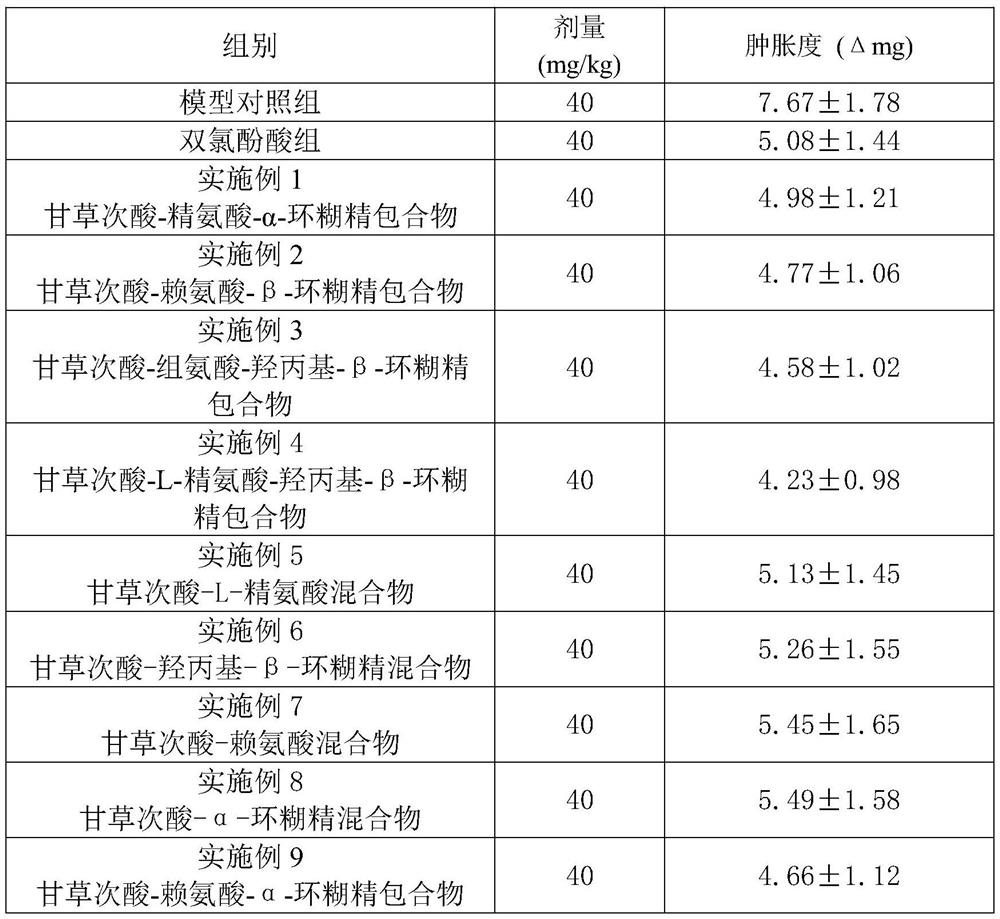
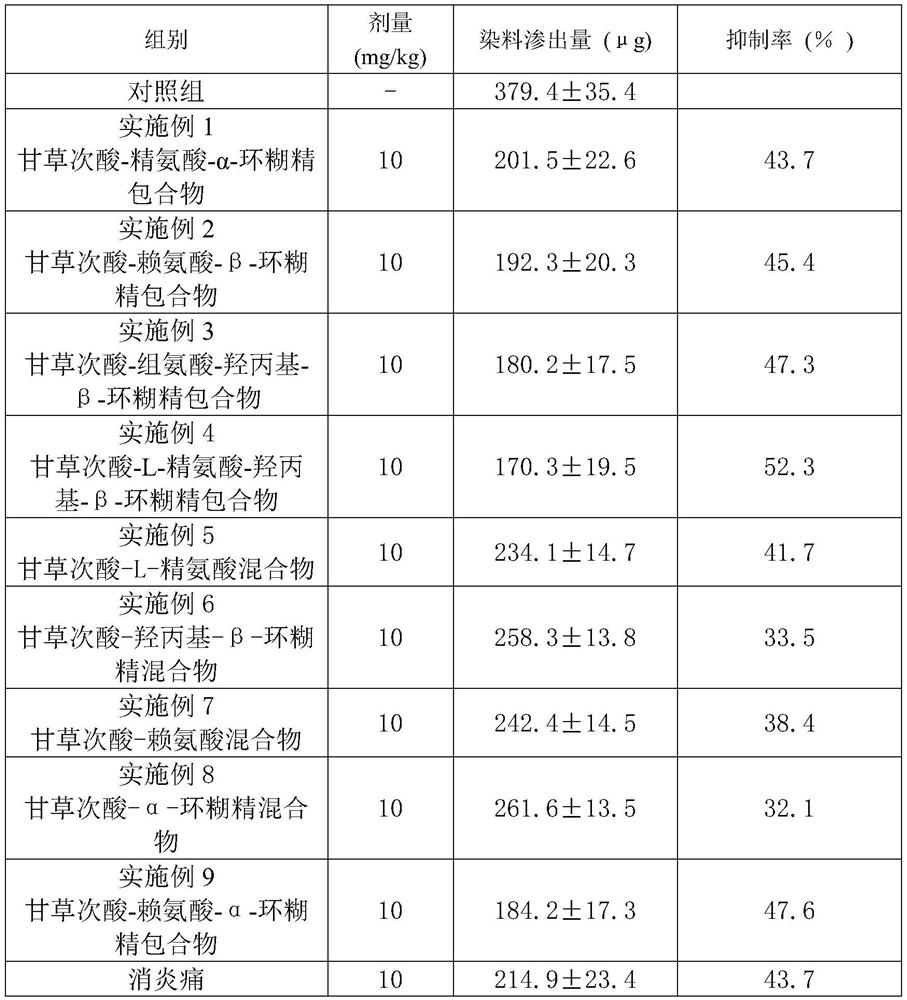
Examples
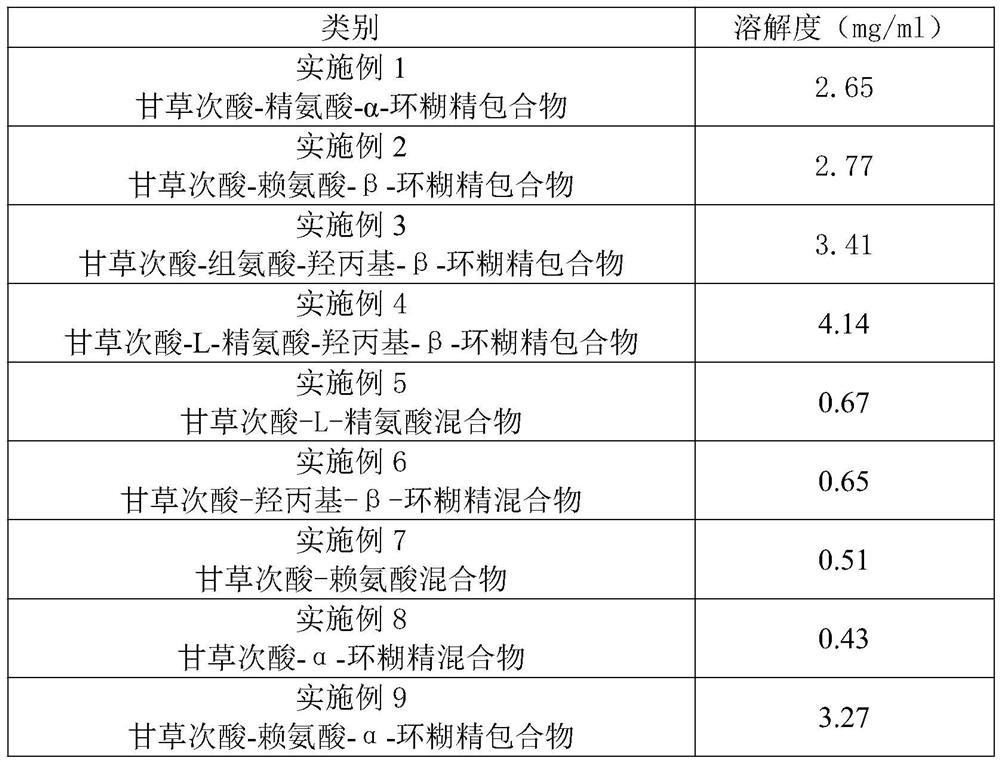
Embodiment 1
[0031] This embodiment provides a water-soluble glycyrrhetinic acid clathrate, and its specific preparation steps are as follows:
[0032] S1. Accurately weigh 100 mg of glycyrrhetinic acid and dissolve it in 10 ml of chloroform to obtain a clear solution 1;
[0033] S2. Accurately weigh 50mg α-cyclodextrin and 50mg arginine and dissolve them in 10ml deionized water to obtain clear solution 2;
[0034] S3. Mix the clear solution 1 and the clear solution 2, and stir at 100 rpm / min for 3 hours at 30 degrees Celsius to obtain a mixed solution;
[0035] S4, the mixed solution is rotated and evaporated for a period of time at 30 degrees Celsius, and the organic solvent is removed to obtain a solid;
[0036] S5. Add 30 ml of water to the solid to dissolve, filter through a 0.45 μm microporous membrane, and freeze-dry at -80° C. for 24 hours to obtain glycyrrhetinic acid-arginine-α-cyclodextrin inclusion compound.
Embodiment 2
[0038] This embodiment provides a water-soluble glycyrrhetinic acid clathrate, and its specific preparation steps are as follows:
[0039] S1. Accurately weigh 50 mg of glycyrrhetinic acid and dissolve it in 20 ml of absolute ethanol to obtain a clear solution 1;
[0040] S2. Accurately weigh 250mg β-cyclodextrin and 100mg lysine and dissolve in 20ml deionized water to obtain clear solution 2;
[0041] S3. Mix the clear solution 1 and the clear solution 2, and stir at 200 rpm / min for 4 hours at 40 degrees Celsius to obtain a mixed solution;
[0042] S4, the mixed solution is rotated and evaporated for a period of time at 40 degrees Celsius, and the organic solvent is removed to obtain a solid;
[0043] S5. Add 100 ml of water to the solid to dissolve, filter through a 0.45 μm microporous membrane, and freeze-dry at -80° C. for 24 hours to obtain glycyrrhetinic acid-lysine-β-cyclodextrin inclusion compound.
Embodiment 3
[0045] This embodiment provides a water-soluble glycyrrhetinic acid clathrate, and its specific preparation steps are as follows:
[0046] S1. Accurately weigh 500 mg of glycyrrhetinic acid and dissolve it in 200 ml of absolute ethanol to obtain a clear solution 1;
[0047] S2. Accurately weigh 5g of hydroxypropyl-β-cyclodextrin and 1g of histidine and dissolve them in 400ml of deionized water to obtain clear solution 2;
[0048] S3. Mix the clear solution 1 and the clear solution 2, and stir at 600 rpm / min for 8 hours at 40 degrees Celsius to obtain a mixed solution;
[0049] S4, the mixed solution is rotated and evaporated for a period of time at 40 degrees Celsius, and the organic solvent is removed to obtain a solid;
[0050] S5. Add 1000ml of water to the solid to dissolve, filter through a 0.45 μm microporous membrane, and spray dry to obtain glycyrrhetinic acid-histidine-hydroxypropyl-β-cyclodextrin inclusion compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com