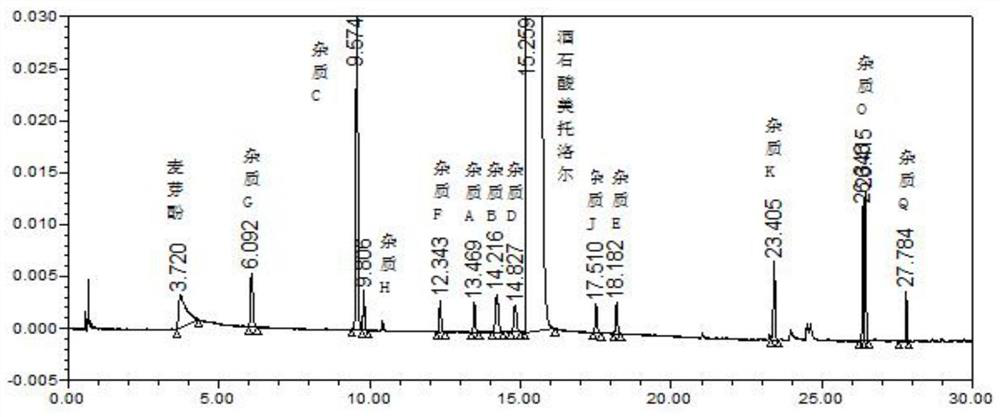
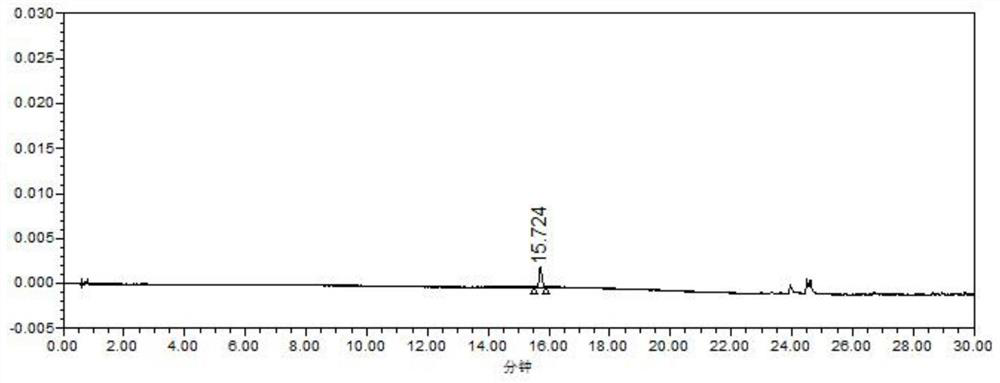
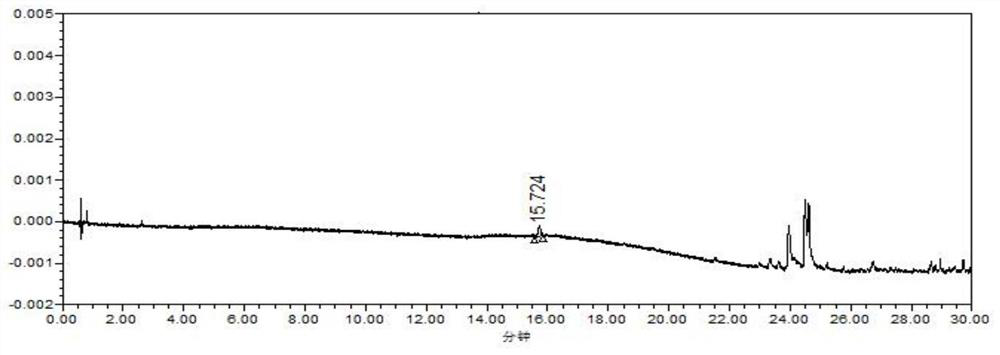
Method for determining metoprolol tartrate and tablet impurities by ultra-high performance liquid chromatography
A technology of metoprolol tartrate and ultra-high performance liquid phase, which is applied in the field of analytical chemistry and can solve problems such as inability to separate and detect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The instrument and chromatographic conditions adopted are as follows:
[0038] (1) Ultra-high performance liquid chromatography: Waters UPLC H-class
[0039] Detector: PDA
[0040] Chromatographic Workstation: Empower
[0041] (2) Chromatographic column: WatersACQUITY BEH C18 column (1.7μm, 2.1mm×100mm)
[0042] (3) Mobile phase A: 0.02 mol / L sodium dihydrogen phosphate, 2% glacial acetic acid mixed buffer solution (pH=5.5) and acetonitrile in a volume ratio of 97:3. Mobile phase B phase: 0.02mol / L sodium dihydrogen phosphate, 2% glacial acetic acid mixed buffer solution (pH=5.5) and acetonitrile in a volume ratio of 30:70.
[0043] (4) Detection conditions
[0044] The mobile phases A and B are eluted according to the following gradient ratios;
[0045] Mobile phase flow rate: 0.4ml / min;
[0046] Column temperature: 35°C
[0047] Detection wavelength: 275nm
[0048] Injection volume: 2μl
[0049] Gradient table: 0 minutes to 3 minutes, mobile phase A is 100%...
Embodiment 2
[0069] The instrument and chromatographic conditions adopted are as follows:
[0070] (1) Ultra-high performance liquid chromatography: Waters UPLC H-class
[0071] Detector: PDA
[0072] Chromatography Workstation: Empower
[0073] (2) Chromatographic column: Waters ACQUITY BEH C18 column (1.7μm, 2.1mm×100mm)
[0074] (3) Mobile phase A phase: 0.015mol / L sodium dihydrogen phosphate, 1.8% glacial acetic acid mixed buffer solution (pH=5.3) and acetonitrile in a volume ratio of 97:3. Mobile phase B phase: 0.015mol / L sodium dihydrogen phosphate, 1.8% glacial acetic acid mixed buffer solution (pH=5.3) and acetonitrile in a volume ratio of 30:70.
[0075] (4) Detection conditions
[0076] The mobile phases A and B are eluted according to the following gradient ratios;
[0077] Mobile phase flow rate: 0.38ml / min;
[0078] Column temperature: 30°C
[0079] Detection wavelength: 273nm
[0080] Injection volume: 1μl
[0081] Gradient table: 0 minutes to 3 minutes, mobile pha...
Embodiment 3
[0086] The instrument and chromatographic conditions adopted are as follows:
[0087] (1) Ultra-high performance liquid chromatography: Waters UPLC H-class
[0088] Detector: PDA
[0089] Chromatography Workstation: Empower
[0090] (2) Chromatographic column: WatersACQUITY BEH C18 column (1.7μm, 2.1mm×100mm)
[0091] (3) Mobile phase A: 0.025 mol / L sodium dihydrogen phosphate, 2.2% glacial acetic acid mixed buffer solution (pH=5.7) and acetonitrile in a volume ratio of 97:3. Mobile phase B phase: 0.025mol / L sodium dihydrogen phosphate, 2.2% glacial acetic acid mixed buffer solution (pH=5.7) and acetonitrile in a volume ratio of 30:70.
[0092] (4) Detection conditions
[0093] The mobile phases A and B are eluted according to the following gradient ratios;
[0094] Mobile phase flow rate: 0.42ml / min;
[0095] Column temperature: 40°C
[0096] Detection wavelength: 277nm
[0097] Injection volume: 5μl
[0098] Gradient table: 0 minutes to 3 minutes, mobile phase A i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com