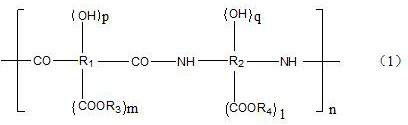
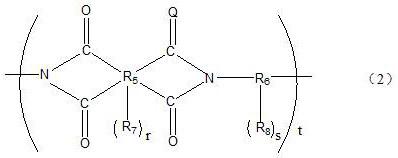
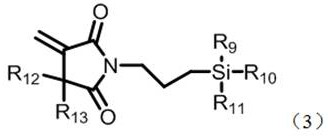
Photosensitive resin composition containing silane coupling agent
A technology of photosensitive resin and silane coupling agent, applied in the field of photosensitive resin composition, can solve the problems of high price of phenylethynyl phthalic anhydride, unsuitable for large-scale industrial use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] The preparation method of the silane coupling agent of general formula (3) is as follows:
[0071] 1) The itaconic anhydride compound represented by formula (4) and the silane compound whose terminal group is amino group represented by formula (5) perform amidation reaction to form amic acid.
[0072] 2) After the amidation reaction, carry out imidization reaction to obtain the silane coupling agent represented by the general formula (3).
[0073]
[0074] In formulas (4) and (5), R 9 , R 10 , R 11 , R 12 , R 13 The definitions are the same as those mentioned above.
[0075] Further, the amidation reaction is carried out in an aprotic polar solvent, and the effect of each aprotic polar solvent is equivalent. Considering the cost and the convenience of acquisition, preferably, the aprotic polar solvent is selected from N-methylpyrrolidone, N,N-dimethylformamide, N,N-dimethylacetamide, dimethyl At least one of sulfoxide and γ-butyrolactone, preferably N-methylpy...
Synthetic example 1
[0100] Synthesis of polyimide resin A-1 (polyamide ester resin):
[0101] Under nitrogen flow, 31.02 g (0.1 mol) of 4,4'-oxydiphthalic anhydride (ODPA), N-methylpyrrolidone ( NMP) 100g, stirred and dissolved at room temperature to obtain a dianhydride solution. Take another three-necked flask equipped with a stirrer, add 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane (BAHF) 29.30g (0.08mol), 1,3-bis(3- Aminopropyl)-1,1,3,3-tetramethyldisiloxane (SiDA) 2.48g (0.01mol) and N-methylpyrrolidone 100g were stirred and dissolved to obtain a diamine solution. Add the diamine solution dropwise to the above-mentioned dianhydride solution, react at room temperature for 1 hour after the dropwise addition, and then react at 50°C for 2 hours. After the reaction was completed, 2.18 g (0.02 mol) of 4-aminophenol was added as an end-capping agent, and reacted at 50° C. for 2 h. Dilute 23.83 g of N,N-dimethylformamide dimethyl acetal with 45 g of NMP, and add the diluted solution dropwise...
Synthetic example 2
[0105] Synthesis of polybenzoxazole resin A-2 (polybenzoxazole precursor resin):
[0106] Under nitrogen flow, add 32.96g (0.09mol) of 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane (BAHF) to a 500mL three-necked flask equipped with a stirrer, dropping funnel and thermometer , 2.18g (0.02mol) of 4-aminophenol, 15.82g (0.2mol) of pyridine, and 100g of N-methylpyrrolidone (NMP) were fully dissolved, and the temperature of the solution was cooled to -15°C. Dissolve 29.51g (0.10mol) of 4,4-diphenyl ether diformyl chloride in 50g of NMP to make a solution, drop the solution into the flask with a dropping funnel, and control the reaction material below 0°C during the dropping process. After the dropwise addition was completed, the stirring reaction was continued for 6 hours under the condition of -10 to -15°C. After the reaction was completed, the reaction solution was poured into 3 L of 10 wt% methanol aqueous solution, and the polymer was precipitated to form a white precipita...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com