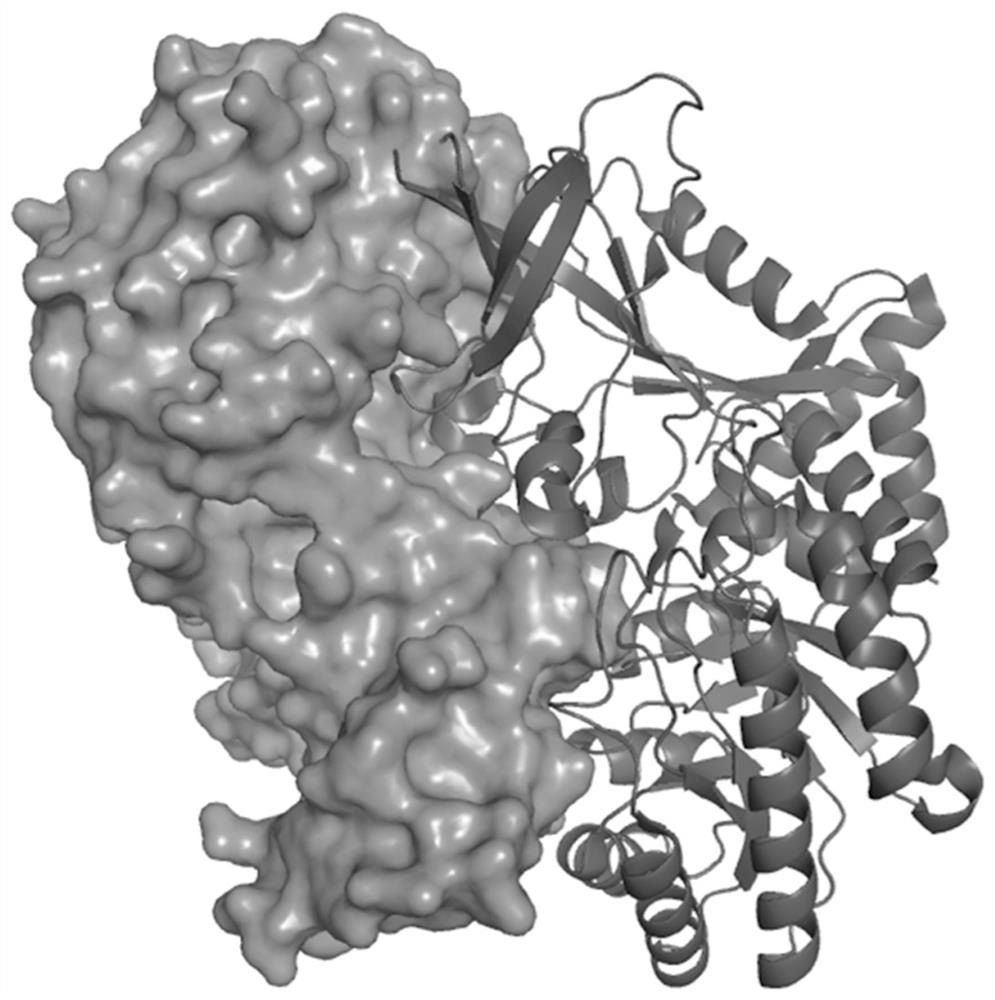
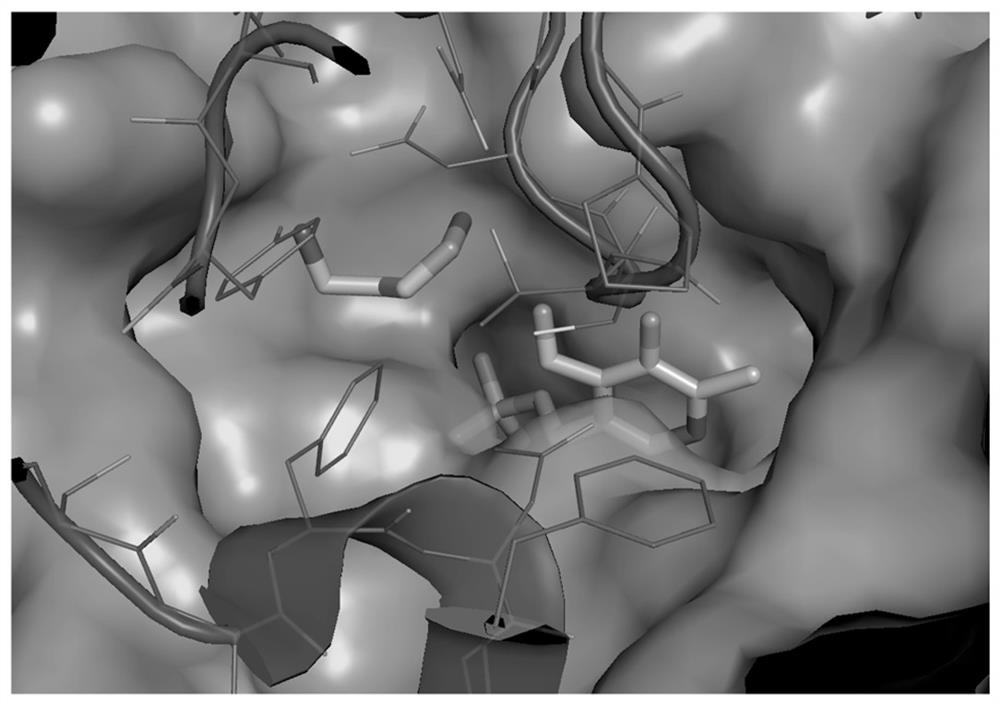
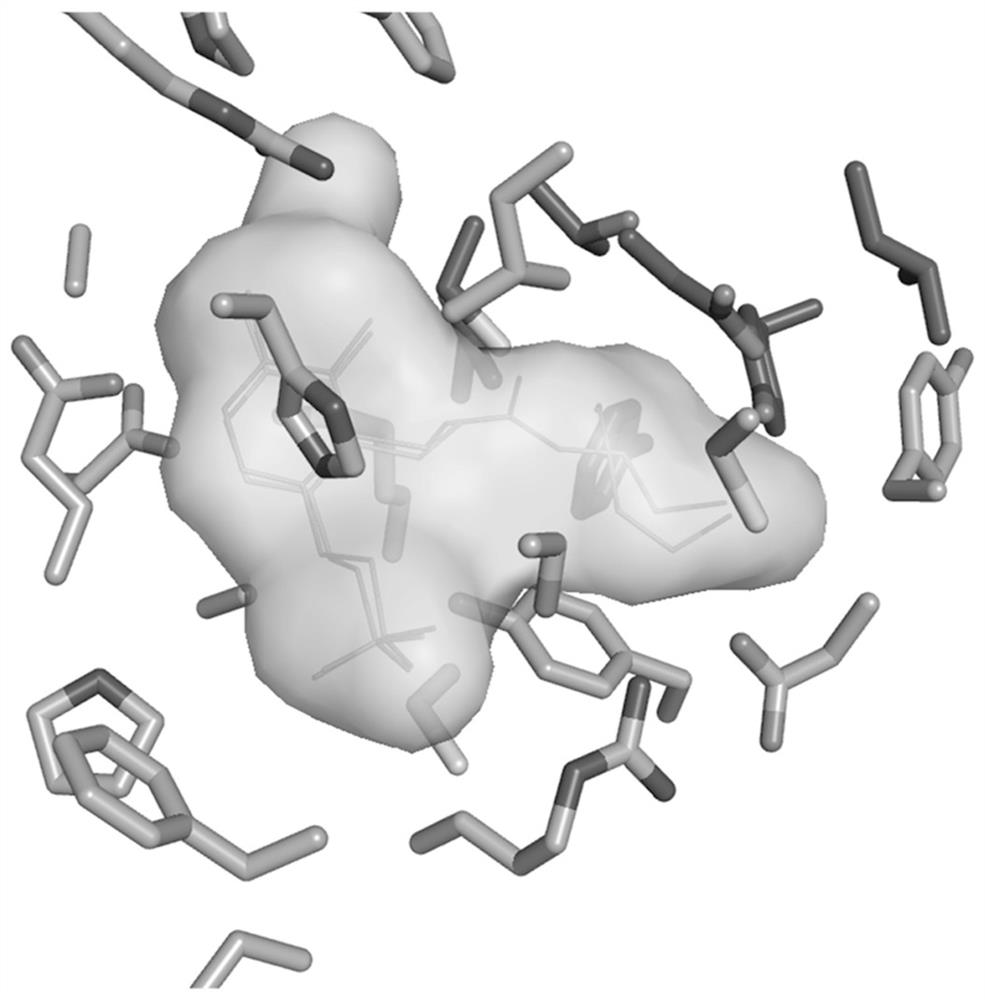
Pharmaceutical application of small-molecule inhibitor in preparation of drugs for inhibiting interaction between ornithine decarboxylase and ornithine decarboxylase antienzyme 1
A small-molecule inhibitor, ornithine decarboxylase technology, is used in anti-tumor drugs, medical preparations containing active ingredients, drug combinations, etc., and can solve problems such as large toxic and side effects, high concentration of action, and weak binding ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Small Molecule Inhibitors: A Chemical Synthetic Route to 6-[N'-(4-Dimethylaminobenzylidene)-hydrazino-[1,3,5]triazole-2,4-diamine.
[0056]
[0057] In a 100ml one-mouth bottle, add D24-1-1 (purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.) 5g, hydrazine hydrate (purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.) (80%wt) 11g and water 50ml , protected by nitrogen, heated to 85°C, reacted for 7h, cooled to room temperature, filtered, and dried in vacuo (45°C, 24h) to obtain 3.85g of white solid D24-1-2 with a yield of 79.4%.
[0058] In a 100ml single-necked bottle, sequentially add 3.5g of D24-1-2, 3.8g of D24-1-3 (purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.) and 40ml of methanol, under nitrogen protection, react at 30°C for 18h, and cool to After filtering at room temperature, the resulting solid was slurried with 20 ml of DMSO, protected by nitrogen, filtered, and dried in vacuo at 45°C for 12 hours to obtain 2.1 ...
Embodiment 2
[0072] The small molecule inhibitor 1,3-dimethyl-8-(2-thienyl)-3,7-dihydro-1H-purine-2,6-dione was purchased from SPECS (http: / / www.specs .net).
[0073] In order to verify whether the small molecule 1,3-dimethyl-8-(2-thienyl)-3,7-dihydro-1H-purine-2,6-dione can inhibit the enzymatic activity of ODC, the Chromatography (HPLC) detects putrescine, the enzyme-catalyzed product, to obtain a half-inhibitory concentration of C small molecules to ODC enzyme activity of about 31.8 μM ( Figure 16 ). Figure 16 In order to detect putrescine, the catalytic product of ODC, by high performance liquid chromatography, the inhibitory efficiency of small molecules on ODC enzyme activity was calculated by comparing with those without adding small molecules.
[0074] For the small molecule 1,3-dimethyl-8-(2-thienyl)-3,7-dihydro-1H-purine-2,6-dione, in order to verify its inhibition of ODC-OAZ1 interaction, A method based on fluorescence resonance energy transfer (FRET) was used. ODC protein...
Embodiment 3
[0080] Small molecule inhibitor: 1-Butyl-N-(4-chlorophenyl)-1H-benzo[d]imidazol-2-amine purchased from SPECS (http: / / www.specs.net).
[0081] In order to verify whether the small molecule 1-butyl-N-(4-chlorophenyl)-1H-benzo[d]imidazol-2-amine can inhibit the enzymatic activity of ODC, the enzyme catalysis was detected by high performance liquid chromatography (HPLC). Product putrescine, the half-inhibitory concentration of C small molecule to ODC enzyme activity is about 31.8 μ M ( Figure 21 ). Figure 21 In order to detect putrescine, the catalytic product of ODC, by high performance liquid chromatography, the inhibitory efficiency of small molecules on ODC enzyme activity was calculated by comparing with those without adding small molecules.
[0082] To verify the inhibition of ODC-OAZ1 interaction by 1-butyl-N-(4-chlorophenyl)-1H-benzo[d]imidazol-2-amine molecules, fluorescence resonance energy transfer (FRET)-based method. ODC protein tagged with YPET fluorescent prote...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com