Identification of lysins and derivatives thereof with bacterial activity against pseudomonas aeruginosa
A lysin, active technology, used in antibacterial drugs, bacterial antigen components, biochemical equipment and methods, etc., can solve problems such as lack of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Example 1. Bacterial Strains and Growth Conditions. Pseudomonas aeruginosa from human blood obtained from the Hospital for Special Surgery in New York (provided by Dr. Lars Westblade, Professor of Pathology and Laboratory Medicine) was used. Antibacterial screening was performed on a clinical isolate of Monocystis bacterium (CFS-1292). Strain CFS-1292 was cultured in lysogenic broth (LB; Sigma-Aldrich), casamino acid (CAA) medium (5 g / L casamino acid, Ameresco / VWR; 5.2 mM K 2 HPO 4 , Sigma-Aldrich; 1 mM MgSO 4 , Sigma-Aldrich) or CAA supplemented with 25% human serum (AB type, male, pooled; Sigma-Aldrich). For the purposes of this disclosure, the particular isolate of P. aeruginosa is not important and commercially available isolates may be used in this experiment.
Embodiment 2
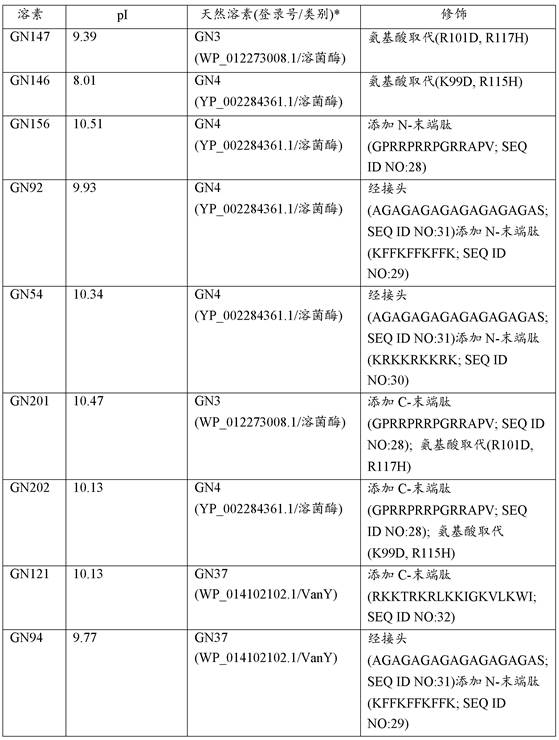
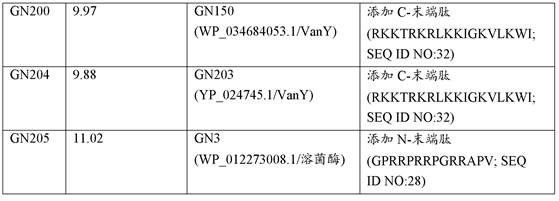
[0125] Example 2. Gene synthesis and cloning. All lysins and modified lysins were synthesized as gBlocks (IDTTechnologies) and cloned into the arabinose-inducible expression vector pBAD24 by overlap extension PCR or by ligation compatible cohesive ends (24) middle. All constructs were transformed into E. coli strain TOP10 (Thermo Fisher Scientific). Other commercially available expression vectors and systems can be used.
Embodiment 3
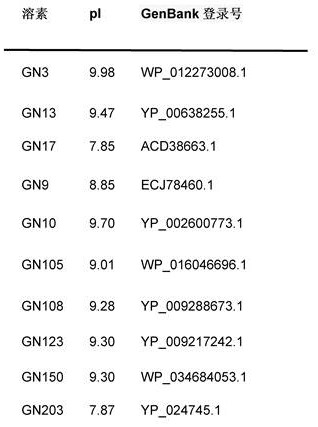
[0126]Example 3. Identification of lysins with intrinsic activity. A set of up to 250 putative lysins and lysin-like enzymes were identified in the GenBank database of Pseudomonas aeruginosa genome sequences. 3 search methods were used: i) a targeted BLASTp screen of all Pseudomonas aeruginosa genomes using query sequences of known lysins, ii) a keyword-based search of all annotated Pseudomonas aeruginosa genomes A search, focusing on all superfamily names associated with lysin (and cell wall hydrolases) catalytic and binding domains; and iii) a visual search for lysin-like genes in phage sequences of non-annotated genomes. Once identified, lysin sequences were synthesized as gBlocks, cloned into pBAD24 and transformed into E. coli TOP10 cells. E. coli clones were then examined in a primary antibacterial activity screen (against live Pseudomonas aeruginosa) using an agar-covered plate-based method (11,13) with modifications to allow the assay to include suspension in 50 mM Tri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com